Abstract
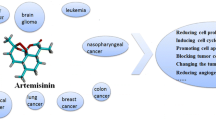
The world is experiencing a cancer epidemic and an increase in the prevalence of the disease. Cancer remains a major killer, accounting for more than half a million deaths annually. There is a wide range of natural products that have the potential to treat this disease. One of these products is artemisinin; a natural product from Artemisia plant. The Nobel Prize for Medicine was awarded in 2015 for the discovery of artemisinin in recognition of the drug’s efficacy. Artemisinin produces highly reactive free radicals by the breakdown of two oxygen atoms that kill cancerous cells. These cells sequester iron and accumulate as much as 1000 times in comparison with normal cells. Generally, chemotherapy is toxic to both cancerous cells and normal cells, while no significant cytotoxicity from artemisinin to normal cells has been found in more than 4000 case studies, which makes it far different than conventional chemotherapy. The pleiotropic response of artemisinin in cancer cells is responsible for growth inhibition by multiple ways including inhibition of angiogenesis, apoptosis, cell cycle arrest, disruption of cell migration, and modulation of nuclear receptor responsiveness. It is very encouraging that artemisinin and its derivatives are anticipated to be a novel class of broad-spectrum antitumor agents based on efficacy and safety. This review aims to highlight these achievements and propose potential strategies to develop artemisinin and its derivatives as a new class of cancer therapeutic agents.




Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- AA:
-
Artemisinic Acid
- A&D:
-
Artemisinin and its derivatives
- ACTs:
-
Artemisinin based combination therapies
- AN production:
-
Artemisinin production
- CDK:
-
Cyclin-dependent kinase
- MVA:
-
Cytosolic Mevalonate Pathway
- DTP:
-
Developmental Therapeutics Program
- DHAA:
-
Dihydroartemisinic Acid
- DW:
-
Dry Weight
- ERK:
-
Extracellular Signal-Regulated Kinases
- MA:
-
Mevalonic Acid
- NCI:
-
National Cancer Institute
- TSTs:
-
Non-glandular T-shaped Trichomes
- ROS:
-
Reactive Oxygen
- SA:
-
Salicylic Acid
- TFAR1:
-
Trichome-specific fatty acyl-CoA reductase 1
- TDZ:
-
Thidiazuron
- TAR1:
-
Trichome and artemisinin regulator 1
- TAFR1:
-
Trichome-Specific Fatty Acyl-CoA Reductase 1
- WHO:
-
World Health Organization
- PLGA:
-
L-lactic-co-glycolic acid
References
WHO (2008) Cancer statistics. WHO, Geneva
Hsiao WLW, Liu L (2010) The role of traditional Chinese herbal medicines in cancer therapy from TCM theory to mechanistic insights. Planta Med 76(11):1118–1131
Hanahan D, Weinberg RA (2011) The hallmarks of cancer: the next generation. Cell 144:646–674
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar C et al (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18:767–773
Efferth T, Saverbrey A, Olbrich A et al (2003) Molecular modes of action of artesunate in tumour cell lines. Mol Pharmacol 64(2):382–394
Efferth T (2003) Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 64(2):382–394
Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO et al (2003) Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 64:382–394
Reungpatthanaphong P, Mankhetkorn S (2002) Modulation of multidrug resistance by artemisinin, artesunate and dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines. Biol Pharm Bull 25(12):1555–1561
Woerdenbag HJ, Moskal TA, Pras N et al (1993) Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod 56(6):849–856
Dondorp AM, Nosten F, Yi P et al (2009) Artemisinin resistance in Plasmodium falciparum malaria. New Engl J Med 361(5):455–467
Gordi T, Lepist EI (2004) Artemisinin derivatives: toxic for laboratory animals, safe for humans? Toxicol Lett 147(2):99–107
Abad MJ, Bedoya LM, Apaza L, Bermejo P (2012) The Artemisia L. genus: a review of bioactive essential oils. Molecules 17(3):2542–2566
Bora KS, Sharma A (2011) The genus Artemisia: a comprehensive review. Pharm Biol 49(1):101–109
Sadiq A, Hayat MQ, Ashraf M (2014) Ethnopharmacology of Artemisia annua L.: A review. Artemisia annua-pharmacology and biotechnology. Springer, Cham, pp 9–25
Lubbe A, Seibert I, Klimkait T, Van der Kooy F (2012) Ethnopharmacology in overdrive: the remarkable anti-HIV activity of Artemisia annua. J Ethnopharmacol 141(3):854–859
Lee SK, Kim H, Park J, Kim H-J, Kim KR, Son SH et al (2017) Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts. Sci Rep 7(1):17332
Li X, Ba Q, Liu Y, Yue Q, Chen P, Li J et al (2017) Dihydroartemisinin selectively inhibits PDGFRα-positive ovarian cancer growth and metastasis through inducing degradation of PDGFRα protein. Cell Discov 3:17042
Feng M-X, Hong J-X, Wang Q, Fan Y-Y, Yuan C-T, Lei X-H et al (2016) Dihydroartemisinin prevents breast cancer-induced osteolysis via inhibiting both breast cancer cells and osteoclasts. Sci Rep 6:19074
Chen H, Sun B, Pan S, Jiang H, Sun X et al (2009) Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs 20:131–140
Chen T, Li M, Zhang R, Wang H et al (2009) Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med 13(7):1358–1370
Kim SJ, Kim MS, Lee JW, Lee CH, Yoo H, Shin SH et al (2006) Dihydroartemisinin enhances radio sensitivity of human glioma cells in vitro. J Cancer Res Clin Oncol 132:129–135
Lu JJ, Chen S, Zhang M, Ding XW, Meng LH et al (2011) The anti-cancer activity of dihydroartemisinin is associated with induction of iron-dependent endoplasmic reticulum stress in colorectal carcinoma HCT116 cells. Invest New Drugs 29:1276–1283
Mu D, Chen W, Yu B, Zhang C, Zhang Y, Qi H et al (2008) Calcium and survivin are involved in the induction of apoptosis by dihydroartemisinin in human lung cancer SPC-A-1 cells. Methods Find Exp Clin Pharmacol 29:33–38
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70:49–56
Zhang Y, Xu G, Zhang S, Wang D, Prabha PS, Zuo Z (2018) Antitumor research on artemisinin and its bioactive derivatives. Nat Prod Bioprospect 8(4):303–319
Roth RJ, Acton N (1989) The isolation of sesquiterpenes from Artemisia annua. J Chem Educ 66:349–350
Brown TA (1993) Gene cloning and DNA analysis and introduction. In: Brown TA (ed), Blackwell Science, pp 139–144
Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ et al (2007) Effects of artemisinin and its derivatives on growth inhibition andapoptosis of oral cancer cells. Head Neck 29:335–340
Posner GH, McRiner AJ, Paik IH, Sur S, Borstnik K, Xie S, Shapiro TA, Alagbala A, Foster B et al (2004) Anticancer and antimalarial efficacy and safety of artemisinin-derived trioxane dimers in rodents. J Med Chem 47:1299–1302
Singh NP, Lai H (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Res 24:2277–2280
Thomas E, Axel S, Armin O, Erich G, Pia R, Oliver W et al (2003) Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 64:382–394
Wu GD, Zhou HJ, Wu XH et al (2006) Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vasc Pharmacol 41(6):205–212
Chen HH, Zhou HJ, Wu GD, Lou XE et al (2004) Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology 71:1–9
Yamachika E, Habte T, Oda D et al (2004) Artemisinin: an alternative treatment for oral squamous cell carcinoma. Anticancer Res 24:2153–2160
Jiao Y, Ge C, Meng Q, Cao J, Tong J, Fan S et al (2007) Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin 28(7):1045–1056. https://doi.org/10.1111/j.1745-7254.2007.00612.x
Disbrow GL, Baege AC, Kierpiec KA et al (2005) Dihydroartemisinin is Cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res 65:10854–10861
Wu JM, Shan F, Wu GS, Li Y, Ding J, Xiao D, Han JX, Atassi G, Leonce S, Caignard DH, Renard P et al (2001) Synthesis and cytotoxicity of artemisinin derivatives containing cyanoarylmethyl group. Eur J Med Chem 36:469–479
Efferth T, Olbrich A, Bauer R et al (2002) mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem Pharmacol 64(4):617–623
Sadava D, Phillips T, Lin C, Kane SE et al (2002) Transferrin overcomes drug resistance to artemisinin in human small-cell lung carcinoma cells. Cancer Lett 179:151–156
Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D et al (2004) Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med 37:998–1009
Efferth T (2004) Combination treatment of glioblastoma multiforme cell lines with the anti-malarial artesunate and the epidermal growth factor receptor tyrosine kinase inhibitor OSI-774. Biochem Pharmacol 67(9):1689–1700
Firestone GL, Sundar SN (2009) Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med 11:32
O’Neill PM, Barton VE, Ward SA et al (2010) The molecular mechanism of action of artemisinin–the debate continues. Molecules 15:1705–1721
Henry L, Tomikazu S, Narendra PS et al (2005) Oncologic, endocrine and metabolic treatment of cancer with artemisinin and artemisinin tagged iron-carrying compounds. Expert Opin Ther Targets 9(5):995–1007
Rowen RJ (2002) Artemisinin: from malaria to cancer treatment. Townsend Lett Doct Patients 86–88
Gong Y, Gallis BM, Goodlett DR, Yang Y, Lu H, Lacoste E et al (2013) Effects of transferrin conjugates of artemisinin and artemisinin dimer on breast cancer cell lines. Anticancer Res 33:123–132
Krishna S, Bustamante L, Haynes RK, Staines HM et al (2008) Artemisinins: their growing importance in medicine. Trends Pharmacol Sci 29:520–527. https://doi.org/10.1016/j.tips.2008.07.004
Paik IH, Xie S, Shapiro TA, Labonte T, Narducci Sarjeant AA, Baege AC et al (2006) Second generation, orally active, antimalarial, artemisinin-derived trioxane dimers with high stability, efficacy, and anticancer activity. JMedChem 49:2731–2734
Sertel S, Plinkert P, Efferth T et al (2013) Activity of artemisinin-type compounds against cancer cells. In: Wagner H, Ulrich-Merzenich G (eds) Evidence and rational based research on chinese drugs. Springer, Vienna, pp 333–362
Stockwin LH, Han B, Yu SX, Hollingshead MG, ElSohly MA, Gul W et al (2009) Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int J Cancer 125:1266–1275
Yoon MK, Mitrea DM, Ou L, Kriwacki RW et al (2012) Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem Soc Trans 40:981–988
Tin AS, Sundar SN, Tran KQ, Park AH, Poindexter KM, Firestone GL et al (2012) Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anticancer Drugs 23:370–379
Chen G, Gong R, Shi X, Yang D, Zhang G et al (2016) Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget 7(31):50302–50314
Huang Z, Huang X, Jiang D, Zhang Y, Huang B, Luo G et al (2016) Dihydroartemisinin inhibits cell proliferation by induced G1 arrest and apoptosis in human nasopharyngeal carcinoma cells. J Cancer Res Ther 12(1):244–247. https://doi.org/10.4103/0973-1482.151855
Tong Y, Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu J, Ou R, Zhang G, Li F, Hu M, Liu Z, Lu L et al (2016) Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget 7(21):31413–31428
Hou J, Wang D, Zhang R, Wang H et al (2008) Experimental therapy of hepatoma with artemisinin and Its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 14(17):5519–5530. https://doi.org/10.1158/1078-0432.CCR-08-0197
Willoughby JA, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL et al (2009) Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem 284(4):2203–2213. https://doi.org/10.1074/jbc.M804491200
Steinbrück G, Pereira I, Efferth T et al (2010) Effects of artesunate on cytokinesis and G2/M cell cycle progression of tumour cells and budding yeast. Cancer Genom Proteom 7(6):337–346
Wang SJ, Gao Y, Chen H, Kong R, Jiang HC, Pan SH, Sun B et al (2010) Dihydroartemisinin inactivates NF-??B and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett 293(1):99–108. https://doi.org/10.1016/j.canlet.2010.01.001
K Liao, J Li, Z Wang et al (2014) Dihydroartemisinin inhibits cell proliferation via AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung cancer cells. Int J Clin Exp Pathol 7(12):8684–8691. Retrieved from www.ijcep.com
Liao K, Li J, Wang Z et al (2014) Dihydroartemisinin inhibits cell proliferation via AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung cancer cells. Int J Clin Exp Pathol 7(12):8684–8691
Xu Q, Li Z-X, Peng H-Q, Sun Z-W, Cheng R-L, Ye Z-M, Li W-X (2011) Artesunate inhibits growth and induces apoptosis in human osteosarcoma HOS cell line in vitro and in vivo. J Zhejiang Univ Sci B 12(4):247–255
Ma H, Yao Q, Zhang AM, Lin S, Wang XX, Wu L, Chen ZT et al (2011) The effects of artesunate on the expression of EGFR and ABCG2 in A549 human lung cancer cells and a xenograft model. Molecules 16(12):10556–10569. https://doi.org/10.3390/molecules161210556
Du W, Pang C, Xue Y, Zhang Q, Wei X et al (2015) Dihydroartemisinin inhibits the Raf/ERK/MEK and PI3K/AKT pathways in glioma cells. Oncol Lett. https://doi.org/10.3892/ol.2015.3699
Zhang J, Ling G, Xia Z, Fengyun D, Liqun L, Zuowang C, Yinghua X, Jiyong L, Qi X, Liu Ju et al (2016) Dihydroartemisinin induces endothelial cell anoikis through the activation of the JNK signaling pathway. Oncol Lett 12:1896–1900
Zhao X, Zhong H, Wang R, Liu D, Waxman S, Zhao L, Jing Y et al (2015) Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget 6(8):5582–5596
Dong F (2014) Dihydroartemisinin targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis. Cancer Biol Ther 15(11):1479–1488
Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW, Jeong HG et al (2010) Suppression of PMA-induced tumor cell invasion by dihydroartemisinin via inhibition of PKCα/Raf/MAPKs and NF-κB/AP-1-dependent mechanisms. Biochem Pharmacol 79(12):1714–1726
Aung W, Sogawa C, Furukawa T, Saga T et al (2011) Anticancer effect of dihydroartemisinin (DHA) in a pancreatic tumor model evaluated by conventional methods and optical imaging. Anticancer Res 31:1549–1558
He Q, Jingxue S, Xiao-Ling S, Jie A, Hong S, Lu W, Ying-Jie H, Qing S, Lin-Chun F, Saeed SM, Ying H et al (2010) Dihydroartemisinin upregulates death receptor 5 expression and cooperates with TRAIL to induce apoptosis in human prostate cancer cells. Cancer Biol Ther 9(10):819–824
Mao H (2013) Involvement of the mitochondrial pathway and Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human breast cancer in vitro. Int J Mol Med 31(1):213–218
Wang JX, Tang W, Shi LP, Wan J, Zhou R, Ni J, Fu YF, Yang YF, Li Y, Zuo JP et al (2007) Investigation of the immunosuppressive activity of artemether on T-cell activation and proliferation. Br J Pharmacol 150:652–661
Wu ZP, Gao CW, Wu YG, Zhu QS, Chen Y, Liu X, Liu C et al (2009) Inhibitive effect of artemether on tumor growth and angiogenesis in the rat C6 orthotopic brain gliomas model. Integr Cancer Ther 8:88–92
Jiao Y, Tong J (2007) Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin 28(7):1045–1056
Cheng R, Li C, Li C, Wei L, Li L, Zhang Y, Gao G et al (2013) The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Invest Ophthalmol Vis Sci 54(5):3400–3409
Yang ND, Shi-Hao T, Shukie N, Yin S, Jing Z, Kevin S, Wei T, Wai-Shiu FW, Han-Ming S (2014) Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J Biol Chem 289(48):33425–33441
Chen H, Shi L, Yang X, Li S, Guo X, Pan L et al (2010) Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int J Hematol 92(4):587–597. https://doi.org/10.1007/s12185-010-0697-3
Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X, Xue D et al (2010) Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J Cancer Res Clin Oncol 136(6):897–903
Jia G, Rui K, Zhi-Bin M, Bing H, Yong-Wei W, Shang-Ha P, Ying-Hua L, Bei S et al (2014) The activation of c-Jun NH2-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J Exp Clin Cancer Res 33:8–15
Thomas S, Quinn BA, Das SK, Dash R, Emdad L, Dasgupta S et al (2013) Targeting the Bcl-2 family for cancer therapy. Exp Opin Ther Targets 17:61–75
Chen Y, Zhang D, Liao Z et al (2015) Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of parkinson’s disease. Mol Neurodegener 10:4
Morrissey C, Gallis B, Solazzi JW, Kim BJ, Gulati R, Vakar-Lopez F, Sasaki T (2010) Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs 21(4):423
Mizushima N, Levine B, Cuervo AM, Klionsky DJ et al (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075
Chen SS, Hu W, Wang Z, Lou XE, Zhou HJ et al (2015) p8 attenuates the apoptosis induced by dihydroartemisinin in cancer cells through promoting autophagy. Cancer Biol Ther. https://doi.org/10.1080/15384047.2015.1026477
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Kroemer G et al (2015) Autophagy in malignant transformation and cancer progression. EMBO J 34(7):856–880. https://doi.org/10.15252/embj.201490784
Chen K, Shou L-M, Lin F, Duan W-M, Wu M-Y, Xie X, Tao M et al (2014) Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anticancer Drugs 25:652–662
Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X et al (2016) Ferroptosis is an autophagic cell death process. Cell Res 26(9):1021–1032. https://doi.org/10.1038/cr.2016.95
Li Z (2016) Artemisinin and its derivatives as a repurposing anticancer agent: what else do we need to do? Molecules 21(10):1331–1339
Wong YK, He Y, Wang J, Xu C, Kalesh KA, Shen H, Wong WSF et al (2017) Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action. Med Res Rev 2017(37):1492–1517. https://doi.org/10.1002/med.21446
Giuliano S, Pagès G (2013) Mechanisms of resistance to anti-angiogenesis therapies. Biochimie 95(6):1110–1119. https://doi.org/10.1016/j.biochi.2013.03.002
Wang SJ, Sun B, Cheng ZX, Zhou HX, Gao Y, Kong R, Bai XW et al (2011) Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by targeting the NF-??B pathway. Cancer Chemother Pharmacol 68(6):1421–1430. https://doi.org/10.1007/s00280-011-1643-7
Boyeva SA, Brezhneva TA, Maltseva AA, Buzlama AV, Slivkin AI (2007) Saponins of the Polemonium caeruleum L. and Beta vulgaris L. plants. The features of obtaining, the comparative assessment of the hypoglycemic activity. Proc Voronezh State Univ Chem Biol Pharm 1:139–141
Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini P-L (2011) 2011: the immune hallmarks of cancer. Cancer Immunol Immunother 60(3):319–326
Efferth T, Albini A, Pfeffer U et al (2006) Microarray expression profiles of angiogenesis-related genes predicttumor cell response to artemisinins. Pharmacogenom J 6:269–278
Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP et al (2017) Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 14(1):11–31. https://doi.org/10.1038/nrclinonc.2016.60
Mi Y, Geng G, Zou Z, Gao J, Luo X, Liu Y et al (2015) Dihydroartemisinin inhibits glucose uptake and cooperates with glycolysis inhibitor to induce apoptosis in non-small cell lung carcinoma cells. PLoS ONE 10(3):1–21. https://doi.org/10.1371/journal.pone.0120426
Ravindra KC, Ho WE, Cheng C, Godoy LC, Wishnok JS, Ong CN, Wong WS, Wogan GN, Tannenbaum SR et al (2015) Untargeted proteomics and systems-based mechanistic investigation of artesunate in human bronchial epithelial cells. Chem Res Toxicol 28(10):1903–1913
Zhou Y, Weichao L, Xiao Y et al (2016) Profiling of Multiple Targets of Artemisinin Activated by Hemin in Cancer Cell Proteome. ACS Chem Biol 11:882–888. https://doi.org/10.1021/acschembio.5b01043
Li Y (2012) Qinghaosu (artemisinin): chemistry and pharmacology. Acta Pharmacol Sin 33:1141–1146. https://doi.org/10.1038/aps.2012.104
Ho WE, Peh HY, Chan TK, Wong WS et al (2014) Artemisinin: pharmacological actions beyond anti-malarial. Pharmacol Ther 142:126–139. https://doi.org/10.1016/j.pharmthera.2013.12.001
Crespo-Ortiz MP, Wei MQ (2012) Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol 2012:247597. https://doi.org/10.1155/2012/247597
Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG et al (2010) Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chem Soc Rev 39(2):435–454
Efferth T (2006) Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets 7:407–421
Reichert S, Reinboldt V, Hehlgans S, Efferth T, Rodel C, Rodel F et al (2012) A radiosensitizing effect of artesunate in glioblastoma cells is associated with a diminished expression of the inhibitor of apoptosis protein survivin. Radiother Oncol 103:394–401. https://doi.org/10.1016/j.radonc.2012.03.018
Michaelis M (2010) Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem Pharmacol 79(2):130–136
Efferth T, Merling A, Krammer PH, Li-Weber M et al (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2(8):693
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR et al (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem 286(8):6587–6601
Zeng QP, Zhang PZ (2011) Artesunate mitigates proliferation of tumor cells by alkylating heme-harboring nitric oxide synthase. Nitric Oxide 24(2):110–112
Du JH, Zhang HD, Ma ZJ, Ji KM et al (2010) Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother Pharmacol 65(5):895–902
Anfosso L, Efferth T, Albini A, Pfeffer U et al (2006) Microarray expression profiles of angiogenesis-related genes predict tumor cell response to artemisinins. Pharmacogenom J 6:269–278
Lai HC, Singh NP, Sasaki T et al (2013) Development of artemisinin compounds for cancer treatment. Invest New Drugs 31(1):230–246
Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L, Sun ZX et al (2007) Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/β-catenin pathway. Int J Cancer 121(6):1360–1365
Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR et al (2015) Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2(5):517–532
Chen HH, Zhou HJ, Fang X et al (2003) Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res 48(3):231–236
Zhou HJ, Wang WQ, Wu GD, Lee J, Li A et al (2007) Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol 47(2):131–138
Youns M, Efferth T, Reichling J, Fellenberg K, Bauer A, Hoheisel JD et al (2009) Gene expression profiling identifies novel key players involved in the cytotoxic effect of Artesunate on pancreatic cancer cells. Biochem Pharmacol 78:273–283
Rasheed SAK, Efferth T, Asangani IA, Allgayer H et al (2010) First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer 127:1475–1485
Li LN, Zhang HD, Yuan SJ, Yang DX, Wang L, Sun ZX et al (2008) Differential sensitivity of colorectal cancer cell lines to artesunate is associated with expression of beta-catenin and E-cadherin. Eur J Pharmacol 588(1):1–8
Lai H, Nakase I, Lacoste E, Singh NP, Sasaki T et al (2009) Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer Res 29(10):3807–3810
Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, Goodlett DR, Tanaka S, Futaki S, Lai H, Sasaki T et al (2009) Transferrin receptor-dependent cytotoxicity of artemisinin–transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett 274(2):290–298
Nakase I, Lai H, Singh NP, Sasaki T et al (2008) Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm 354:28–33
Singh NP, Verma K (2002) Case report of a laryngeal squamous cell carcinoma treated with artesunate. Arch Oncol 10(4):279–280
Berger TG, Dieckmann D, Efferth T, Schultz ES, Funk JO, Baur A, Schuler G et al (2005) Artesunate in the treatment of metastatic uveal melanoma-first experiences. Oncol Rep 14:1599–1604
Zhang Z, Yu SQ, Miao LY, Huang XY, Zhang XP, Zhu YP, Xia XH, Li DQ et al (2008) Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao = J Chin Integr Med 6(2):134–138
Lu JJ, Meng LH, Shankavaram UT, Zhu CH, Tong LJ, Chen G, Lin LP, Weinstein JN, Ding J et al (2010) Dihydroartemisinin accelerates c-MYC oncoprotein degradation and induces apoptosis in c-MYC-overexpressing tumor cells. Biochem Pharmacol 80(1):22–30
Lu YY, Chen TS, Qu JL, Pan WL, Sun L, Wei XB et al (2009) Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed Sci 16:16–20
Lu YY, Chen TS, Wang XP, Qu JL, Chen M et al (2010) The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett 584:4019–4026
Riganti C, Doublier S, Viarisio D, Miraglia E, Pescarmona G, Ghigo D et al (2009) Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1αand P-glycoprotein overexpression. Br J Pharmacol 156:1054–1066
D’Alessandro S, Basilico N, Corbett Y, Scaccabarozzi D, Omodeo-Sale F, Saresella M, Marventano I, Vaillant M, Olliaro P, Taramelli D et al (2011) Hypoxia modulates the effect of dihydroartemisinin on endothelial cells. Biochem Pharmacol 82:476–484
Zhang J, Li L, Liu X, Wang Y, Zhao D et al (2012) Study on chemical constituents of Artemisia sphaerocephala. China J Chin Materia Med 37(2):238–242
Zhang CZ, Zhang H, Yun J, Chen GG, Lai PB et al (2012) Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol 83(9):1278–1289
Wang SJ (2010) Dihydroartemisinin inactivates NF-??B and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett 293(1):99–108
Wang SJ (2011) Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by targeting the NF-??B pathway. Cancer Chemother Pharmacol 68(6):1421–1430
Wu XL, Zhang WG, Shi XM, An P, Sun WS, Qiao CL, Wang Z et al (2011) Effect of artemisinin on the expressions of GRalpha mRNA, GRbeta mRNA and P300/CBP protein in lupus nephritis mice. J Chin Med Mater 17(4):277–282
Wang J, Zhang C-J, Chia WN et al (2015) Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat Commun 6:10111
D’Alessandro S, Gelati M, Basilico N, Parati EA, Haynes RK, Taramelli D et al (2007) Differential effects on angiogenesis of two antimalarial compounds, dihydroartemisinin and artemisone: implications for embryotoxicity. Toxicology 241(1):66–74
Wei-Ling X, Pei-Hui Y, Zeng X, Ji-Ye C et al (2009) Effect of 4-(12-dihydroartemisininoxy) benzoic acid hydrazide transferrin tagged drug on human breast cancer cells. Chin J Anal Chem 37(5):671–675
Singh NP, Panwar VK (2006) Case report of a pituitary macroadenoma treated with artemether. Integr Cancer Ther 5:391–394
Farsam V, Hassan ZM, Zavaran-Hosseini A, Noori S, Mahdavi M, Ranjbar M et al (2011) Antitumor and immunomodulatory properties of artemether and its ability to reduce CD4+ CD25+ FoxP3+ T reg cells in vivo. Int Immunopharmacol 11:1802–1808
Alcântara DDFÁ, Ribeiro HF, Cardoso PCS, Araújo TMT, Burbano RR, Guimarães AC, Khayat AS, Oliveira BM et al (2013) In vitro evaluation of the cytotoxic and genotoxic effects of artemether, an antimalarial drug, in a gastric cancer cell line (PG100). J Appl Toxicol 33:151–156
Zhao Y, Jiang W, Li B, Yao Q, Dong J, Chen Y, Pan X, Li J, Zheng J, Pang X et al (2011) Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing no production to induce cell cycle arrest at G 2/M phase. Int Immunopharmacol 11:2039–2046
Efferth T, Oesch F (2004) Oxidative stress response of tumor cells: microarray-based comparison between artemisinins and anthracyclines. Biochem Pharmacol 68:3–10
Luthria DL (2006) Significance of sample preparation in developing analytical methodologies for accurate estimation of bioactive compounds in functional foods. J Sci Food Agric 86(14):2266–2272
Castellano G, Tena J, Torrens F et al (2012) Classification of phenolic compounds by chemical structural indicators and its relation to antioxidant properties of posidonia oceanica (L.) Delile. Environment 2:6–9
Ferreira JF, Luthria DL, Sasaki T, Heyerick A et al (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15(5):3135–3170
Croteau R, Kutchan T, Lewis N et al (2000) Natural products (secondary metabolites). Biochem Mol Biol Plants 24:1250–1319
Debeaujon I, Peeters AJ, Léon-Kloosterziel KM, Koornneef M et al (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13(4):853–871
Kitamura S (2006) Transport of flavonoids: from cytosolic synthesis to vacuolar accumulation. The science of flavonoids. Springer, Cham, pp 123–146
Grassi D, Desideri G, Ferri C et al (2010) Flavonoids: antioxidants against atherosclerosis. Nutrients 2:889–902
Middleton JR (1998) Effect of plant flavonoids on immune and inflammatory cell function. Flavonoids in the living system. Springer, Cham, pp 175–182
Falcone Ferreyra ML, Rius S, Casati P et al (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222
Aberham A, Cicek SS, Schneider P, Stuppner H et al (2010) Analysis of sesquiterpene lactones, lignans, and flavonoids in wormwood (Artemisia absinthium L.) using high-performance liquid chromatography (HPLC)—mass spectrometry, reversed phase HPLC, and HPLC—solid phase extraction—nuclear magnetic resonance. J Agric Food Chem 58(20):10817–10823
Singh R, Verma PK, Singh G et al (2012) Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J Intercult Ethnopharmacol 1(2):101–104
Hajdú Z, Martins A, Orbán-Gyapai O, Forgo P, Jedlinszki N, Máthé I, Hohmann J et al (2014) Xanthine oxidase-inhibitory activity and antioxidant properties of the methanol extract and flavonoids of Artemisia asiatica. Rec Nat Prod 8(3):299
Qnais E, Raad D, Bseiso Y et al (2014) Analgesic and anti-inflammatory effects of an extract and flavonoids from Artemisia herba-alba and their mechanisms of action. Neurophysiology 46(3):238–246
Suresh J, Ahuja J, Paramakrishnan N, Sebastian M et al (2012) Total phenolic and total flavonoids content of aerial parts of Artemisia abrotanum Linn. and A. pallens wall. Anal Chem Lett 2(3):186–191
Xiao M, Luo D, Ke Z, Ye J, Tu PF (2014) A novel polyacetylene from the aerial parts of Artemisia lactiflora. Phytochem Lett 8:52–54
Mabeza GF, Loyevsky M, Gordeuk VR, Weiss G et al (1999) Iron chelation therapy for malaria: a review. Pharmacol Ther 81(1):53–75
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett 91(1):41–46
Moore JC, Lai H, Li JR, Ren RL, McDougall JA, Singh NP, Chou CK et al (1995) Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett 98(1):83–87
Li XY, Zhao Y, Sun MG, Shi JF, Ju RJ, Zhang CX, Lu WL et al (2014) Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials 35(21):5591–5604. https://doi.org/10.1016/j.biomaterials.2014.03.049
Li P, Yang S, Dou M, Chen Y, Zhang J, Zhao X et al (2014) Synergic effects of artemisinin and resveratrol in cancer cells. J Cancer Res Clin Oncol 140(12):2065–2075
Dilshad E, Zafar S, Ismail H, Waheed MT, Cusido RM, Palazon J, Mirza B (2016) Effect of rol genes on polyphenols biosynthesis in Artemisia annua and their effect on antioxidant and cytotoxic potential of the plant. Appl Biochem Biotechnol 179(8):1456–1468
Dilshad E, Ismail H, Khan MA, Cusido RM, Mirza B (2020) Metabolite profiling of Artemisia carvifolia Buch transgenic plants and estimation of their anticancer and antidiabetic potential. Biocatal Agric Biotechnol 24:101539
Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, Dusonchet L, Gebbia N, Abbadessa V, Crosta L et al (2005) Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol 37(8):1709–1726
Yeon J, Raj C, Thapa K, Soon C, Jong Y, Kim O et al (2016) Nanoparticle-based combination drug delivery systems for synergistic cancer treatment. J Pharm Investig. https://doi.org/10.1007/s40005-016-0252-1
Ochiuz L, Grigoras C, Popa M, Stoleriu I, Munteanu C, Timofte D, Grigoras AG et al (2016) Alendronate-loaded modified drug delivery lipid particles intended for improved oral and topical administration. Molecules 21(7):858. https://doi.org/10.3390/molecules21070858
Gharib A, Faezizadeh Z, Mesbah-Namin SA, Saravani R et al (2014) Preparation, characterization and in vitro efficacy of magnetic nanoliposomes containing the artemisinin and transferrin. Daru 22(1):44–50. https://doi.org/10.1186/2008-2231-22-44
Wu GS (2013) Synergistic anti-cancer activity of the combination of dihydroartemisinin and doxorubicin in breast cancer cells. Pharmacol Rep 65(2):453–459
Eltayeb SE, Su Z, Xiao Y, Ping Q et al (2016) Antitumor activity of transferrin-modified- artemether lipid nanospheres in cancer cell lines. J Drug Deliv Sci Technol 31:118–129. https://doi.org/10.1016/j.jddst.2015.12.003
Nguyen DX, Massague J (2014) Genetic determinants of cancer metastasis. Nat Rev Genet 8:341–352
Jiang W, Huang Y, Wang J, Yu X, Zhang L et al (2013) The synergistic anticancer effect of artesunate combined with allicin in osteosarcoma cell line in vitro and in vivo. Asian Pac J Cancer Prev 14:4615–4619
Krusche B, Arend J, Efferth T et al (2013) Synergistic inhibition of angiogenesis by artesunate and captopril in vitro and in vivo. Evid Based Complement Altern Med. https://doi.org/10.1155/2013/454783
Acknowledgements
Dr. John Suberu, Department of Chemical Engineering and Biotechnology, University of Cambridge, is acknowledged for the proof reading of the paper.
Funding
Funding information is not applicable/No funding was received.
Author information
Authors and Affiliations
Contributions
BHK conceived and designed the study. BHK, WKK, AUK, HI and ED wrote the manuscript. BM reviewed the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any competing interests.
Ethical approval
No ethical considerations apply for this review paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kiani, B.H., Kayani, W.K., Khayam, A.U. et al. Artemisinin and its derivatives: a promising cancer therapy. Mol Biol Rep 47, 6321–6336 (2020). https://doi.org/10.1007/s11033-020-05669-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05669-z