Abstract
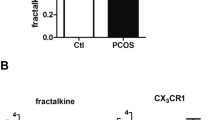
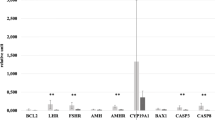
Owing to the role of fractalkine in regulating cellular apoptosis/proliferation, we investigated fractalkine effects on apoptosis/proliferation signaling of granulosa cells in polycystic ovarian syndrome (PCOS) patients through in vitro and in vivo experiments. In vivo, granulosa cells were collected from 40 women undergoing oocyte retrieval (20 controls and 20 PCOS). The expression levels of fractalkine, BAX, Bcl2, Bcl2-XL, Bad, and TNF-α were assessed using RT-PCR. In vitro, we determined the effect of different doses of fractalkine on the expression of the above mentioned genes in GCs of both groups. We found that the expression levels of fractalkine and Bcl-2 were significantly lower in the GCs of PCOS patients compared to the control group (p < 0.05). In contrast, the expression levels of TNF-α and BAX were higher in the patient's group than in the control group. The results suggested that expression levels of fractalkine were negatively and positively correlated with the number of oocytes and fertilized oocytes respectively. Moreover, fractalkine could dose-dependently increase fractalkine and decrease BAD, BAX, Bcl-xl, and TNF-α expressions in the control GCs. In contrast, GCs collected from PCOS patients revealed an increase in expression of BAD, BAX, and Bcl-xl following fractalkine treatment. Our findings indicated that insufficient expression of fractalkine in PCOS patients is related with elevated apoptotic and inflammatory markers and reduced anti-apoptotic genes in the GCs.




Similar content being viewed by others
References
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan R (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106(1):6–15
Diao F-Y, Xu M, Hu Y, Li J, Xu Z, Lin M, Wang L, Zhou Y, Zhou Z, Liu J (2004) The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J Mol Endocrinol 33(1):59–72
Rutkowska AZ, Diamanti-Kandarakis E (2016) Polycystic ovary syndrome and environmental toxins. Fertil Steril 106(4):948–958
Muralidhara KD, Adhikari PM, Muralidhara D (2015) A study on the pattern of genetic inheritance of polycystic ovarian syndrome. Br J Med Med Res 5(10):1230
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7(4):219
Asselin E, Xiao CW, Wang YF, Tsang BK (2000) Mammalian follicular development and atresia: role of apoptosis. Neurosignals 9(2):87–95
Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A (2008) Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab 93(3):881–887
Salehi E, Aflatoonian R, Moeini A, Yamini N, Asadi E, Khosravizadeh Z, Tarzjani MD, Abolhassani F (2017) Apoptotic biomarkers in cumulus cells in relation to embryo quality in polycystic ovary syndrome. Arch Gynecol Obstet 296(6):1219–1227
Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2(11):838
Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ (2000) The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol 165(1):397–403
White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR (2009) Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res 85(4):825–835
Ben-Shlomo I, Rauch R, Avsian-Kretchmer O, Hsueh AJ (2007) Matching receptome genes with their ligands for surveying paracrine/autocrine signaling systems. Mol Endocrinol 21(8):2009–2014
Zhao P, De A, Hu Z, Li J, Mulders SM, Sollewijn Gelpke MD, Duan E-K, Hsueh AJ (2008) Gonadotropin stimulation of ovarian fractalkine expression and fractalkine augmentation of progesterone biosynthesis by luteinizing granulosa cells. Endocrinology 149(6):2782–2789
Huang S, Pang Y, Yan J, Lin S, Zhao Y, Lei L, Yan L, Li R, Ma C, Qiao J (2016) Fractalkine restores the decreased expression of StAR and progesterone in granulosa cells from patients with polycystic ovary syndrome. Sci Rep 6:26205
Yousefi S, Soleimanirad J, Hamdi K, Farzadi L, Ghasemzadeh A, Kazemi M, Mahdipour M, Rahbarghazi R, Nouri M (2018) Distinct effect of fetal bovine serum versus follicular fluid on multipotentiality of human granulosa cells in in vitro condition. Biologicals 52:44–48
Aghadavod E, Zarghami N, Farzadi L, Zare M, Barzegari A, Movassaghpour AA, Nouri M (2015) Isolation of granulosa cells from follicular fluid; applications in biomedical and molecular biology experiments. Adv Biomed Res 4:250
Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S (2005) Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod 20(2):373–381
Nagashimada M, Ni Y, Ota T (2018) Loss of fractalkine-CX3CR1 signaling exacerbates obesity-induced adipose tissue inflammation and insulin resistance through M1 dominant shift in macrophages. Diabetes. https://doi.org/10.2337/db18-1989-P
Riopel M, Seo JB, Bandyopadhyay GK, Li P, Wollam J, Chung H, Jung S-R, Murphy A, Wilson M, de Jong R (2018) Chronic fractalkine administration improves glucose tolerance and pancreatic endocrine function. J Clin Invest. https://doi.org/10.1172/JCI94330
Zhu Q, Zuo R, He Y, Wang Y, Chen Z-J, Sun Y, Sun K (2016) Local regeneration of cortisol by 11β-HSD1 contributes to insulin resistance of the Granulosa cells in PCOS. J Clin Endocrinol Metab 101(5):2168–2177
Reyes-Muñoz E, Sathyapalan T, Rossetti P, Shah M, Long M, Buscema M, Valenti G, La Rosa VL, Cianci S, Vitale SG (2018) Polycystic ovary syndrome: implication for drug metabolism on assisted reproductive techniques—a literature review. Adv Ther 35(11):1805–1815
Laganà AS, Rossetti P, Sapia F, Chiofalo B, Buscema M, Valenti G, Rapisarda AMC, Vitale SG (2017) Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: changing the perspective of the disease. Int J Endocrinol Metab 15(1):e43695
Laganà AS, Garzon S, Casarin J, Franchi M, Ghezzi F (2018) Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab 29:768
Guo X, Pan Y, Xiao C, Wu Y, Cai D, Gu J (2012) Fractalkine stimulates cell growth and increases its expression via NF-κ B pathway in RA-FLS. Int J Rheum Dis 15(3):322–329
Wang H, Cai J, Du S, Guo Z, Xin B, Wang J, Wei W, Shen X (2017) Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochem Funct 35(6):315–326
Fogarty CE, Bergmann A (2015) The sound of silence: signaling by apoptotic cells. In: Current topics in developmental biology, vol 114. Elsevier, Amsterdam, pp 241–265
Sun C, Qiao J, Gao D (2006) The expression of apoptosis-related protein Bcl-2, Bax, P53 and PDCD5 in granulosa cells of the PCOS. Chin J Clin Obstetr Gynecol
Yeh J, Kim HH (1996) Polycystic ovary syndrome (PCOS): the possible roles of apoptosis in human granulosa cells. In: Polycystic ovary syndrome. Springer, New York pp. 51 70
Onalan G, Selam B, Baran Y, Cincik M, Onalan R, Gündüz U, Ural AU, Pabuccu R (2005) Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod 20(9):2391–2395
Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang Y, Liu S, Tan J, Yan Q (2017) ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis 8(10):e3145
Ding L, Gao F, Zhang M, Yan W, Tang R, Zhang C, Chen Z-J (2016) Higher PDCD4 expression is associated with obesity, insulin resistance, lipid metabolism disorders, and granulosa cell apoptosis in polycystic ovary syndrome. Fertil Steril 105(5):1330–1337
Yu YS, Sui HS, Han ZB, Wei L, Luo MJ, Tan JH (2004) Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res 14(4):341
Wu X-Q, Wang Y-Q, Xu S-M, Liu J-F, Bi X-Y, Wang Z-Q, Zhang J-P (2017) The WNT/β-catenin signaling pathway may be involved in granulosa cell apoptosis from patients with PCOS in North China. J Gynecol Obstetr Hum Reprod 46(1):93–99
Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Feng L, Melby PC (2003) Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J 373(2):547–558
White GE, Greaves DR (2012) Fractalkine: a survivor's guide: chemokines as antiapoptotic mediators. Arterioscler Thromband Vasc Biol 32(3):589–594
Vetvicka V, Lagana AS, Salmeri FM, Triolo O, Palmara VI, Vitale SG, Sofo V, Králíčková M (2016) Regulation of apoptotic pathways during endometriosis: from the molecular basis to the future perspectives. Arch Gynecol Obstet 294(5):897–904
Laganà AS, Vitale SG, Salmeri FM, Triolo O, Frangež HB, Vrtačnik-Bokal E, Stojanovska L, Apostolopoulos V, Granese R, Sofo V (2017) Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses 103:10–20
Long M, Zhou J, Li D, Zheng L, Xu Z, Zhou S (2015) Long-term over-expression of neuropeptide Y in hypothalamic paraventricular nucleus contributes to adipose tissue insulin resistance partly via the Y5 receptor. PLoS ONE 10(5):e0126714
Nekoonam S, Naji M, Nashtaei MS, Mortezaee K, Koruji M, Safdarian L, Amidi F (2017) Expression of AKT1 along with AKT2 in granulosa-lutein cells of hyperandrogenic PCOS patients. Arch Gynecol Obstet 295(4):1041–1050
Restuccia DF, Hynx D, Hemmings BA (2012) Loss of PKBβ/Akt2 predisposes mice to ovarian cyst formation and increases the severity of polycystic ovary formation in vivo. Dis Models Mech 5(3):403–411
Liu Q, Li Y, Feng Y, Liu C, Ma J, Li Y, Xiang H, Ji Y, Cao Y, Tong X (2016) Single-cell analysis of differences in transcriptomic profiles of oocytes and cumulus cells at GV, MI, MII stages from PCOS patients. Sci Rep 6:39638
Lan C-W, Chen M-J, Tai K-Y, Danny C, Yang Y-C, Jan P-S, Yang Y-S, Chen H-F, Ho H-N (2015) Functional microarray analysis of differentially expressed genes in granulosa cells from women with polycystic ovary syndrome related to MAPK/ERK signaling. Sci Rep 5:14994
Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, Abbasi M (2016) Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet 294(1):185–192
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117(4):421–426
Delbes G, Hales BF, Robaire B (2009) Toxicants and human sperm chromatin integrity. MHR: Basic science of reproductive medicine 16 (1):14–22
Castilla J, Zamora S, Gonzalvo M, Del Castillo JL, Roldan-Nofuentes J, Clavero A, Björndahl L, Martínez L (2010) Sperm chromatin structure assay and classical semen parameters: systematic review. Reprod Biomed Online 20(1):114–124
Ward WS (2009) Function of sperm chromatin structural elements in fertilization and development. MHR 16(1):30–36
Zini A (2011) Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 57(1–2):78–85
Bungum M, Bungum L, Giwercman A (2011) Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 13(1):69
Paoli D, Lombardo F, Lenzi A, Gandini L (2014) Sperm cryopreservation: effects on chromatin structure. In: Genetic damage in human spermatozoa. Springer, Berlin, pp 137–150
Björndahl L, Kvist U (2014) Structure of chromatin in spermatozoa. In: Genetic damage in human spermatozoa. Springer, New York, pp 1–11
Steger K, Balhorn R (2018) Sperm nuclear protamines: a checkpoint to control sperm chromatin quality. Anat Histol Embryol 47(4):273–279
Acknowledgments
We thank staff of Al-Zahra Hospital of Tabriz and Milad Fertility Center for providing the patients. Some of the data included are part of the M.Sc. thesis of Aydin Raei Sadigh.
Funding
This study was funded by the Stem Cell Research Center, Tabriz University of Medical Sciences [Grant Number: 57778].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Tabriz University of Medical (Approval cod: IR.TBZMED.REC.1396.181).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raei Sadigh, A., Darabi, M., Salmassi, A. et al. Fractalkine and apoptotic/anti-apoptotic markers in granulosa cells of women with polycystic ovarian syndrome. Mol Biol Rep 47, 3593–3603 (2020). https://doi.org/10.1007/s11033-020-05452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05452-0