Abstract
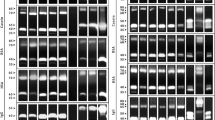
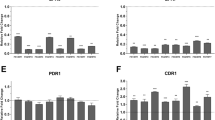
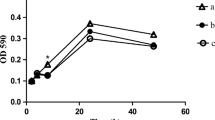
Candida albicans infection development depends on several factors associated with this etiological agent, including secreted aspartyl protease (Sap) production. Sap expression commonly occurs under selective pressure caused by the presence of antifungals. Fluconazole is the main antifungal drug used for treatment or prophylaxis. This study investigated the influence of inhibitory and sub-inhibitory fluconazole concentrations on Sap activity and their gene transcription for three clinical C. albicans isolates. Two isolates presented significant increases in Sap activity and transcription of SAP 1-8 genes in the presence of 1 MIC80 of fluconazole compared to the absence of the antifungal agent. The results suggest that the increase in Sap activity occurs due to an upregulation of the SAP gene transcription influenced by fluconazole. This suggests the importance of all SAP genes in the progression of bloodstream infections compared to primary tissue infection. However, this phenomenon does not occur everywhere, and it is multifactorial. This may be related to the selective pressure effect on transcription modulators. Although preliminary, these results open a new perspective for the study of virulence factors.

Similar content being viewed by others
References
Barnes RA (2008) Early diagnosis of fungal infection in immunocompromised patients. J Antimicrob Chemother 61:i3–i6. https://doi.org/10.1093/jac/dkm424
Liss B, Vehreschild JJ, Bangard C, Maintz D, Frank K, Gronke S, Michels G, Hamprecht A, Wisplinghoff H, Markiefka B, Hekmat K, Vehreschild MJGT, Cornely OA (2015) Our 2015 approach to invasive pulmonary aspergillosis. Mycoses 58(6):375–382
McCullough MJ, Ross BC, Reade PC (1996) Candida albicans: a review of its history, taxonomy, epidemiology, virulence attributes, and methods of strain differentiation. Int J Oral Maxillofac Surg 25:136–144. https://doi.org/10.1016/S0901-5027(96)80060-9
McManus BA, Coleman DC (2014) Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect Genet Evol 21:166–178. https://doi.org/10.1016/j.meegid.2013.11.008
Giacomazzi J, Baethgen L, Carneiro LC, Millington MA, Denning DW, Colombo AL, Pasqualotto AC (2016) The burden of serious human fungal infections in Brazil. Mycosis 59:145–150. https://doi.org/10.1111/myc.12427
Ruhnke M (2006) Epidemiology of Candida albicans infections and role of non-Candida albicans yeasts. Curr Drug Targets 7:495–504. https://doi.org/10.2174/138945006776359421
Guinea J (2014) Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20:5–10. https://doi.org/10.1111/1469-0691.12539
Doi AM, Pignatari ACC, Edmond MB, Marra AR, Camargo LFA, Siqueira RA, da Mota VP, Colombo AL (2016) Epidemiology and microbiologic characterization of nosocomial candidemia from a Brazilian National Surveillance Program. PLoS ONE 11:e0146909. https://doi.org/10.1371/journal.pone.0146909
Akpan A, Morgan R (2002) Oral candidiasis. Postgrad Med J 78:455–459. https://doi.org/10.1136/pmj.78.922.455
Herwald SE, Kumamoto CA (2014) Candida albicans niche specialization: features that distinguish biofilm cells from commensal cells. Curr Fungal Infect Rep 8:179–184. https://doi.org/10.1007/s12281-014-0178-x
Sobel JD (2007) Vulvovaginal candidosis. Lancet 369:1961–1971. https://doi.org/10.1016/S0140-6736(07)60917-9
Giolo MP, Svidzinski TIE (2010) Phisiopathogenesis, epidemiology and laboratory diagnosis of candidemia. J Bras Patol Med Lab 46:225–234. https://doi.org/10.1590/S1676-24442010000300009
Papon N, Courdavault V, Clastre M, Bennett RJ (2013) Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog 9:e1003550. https://doi.org/10.1371/journal.ppat.1003550
Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MJSM (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62:10–24. https://doi.org/10.1099/jmm.0.045054-0
Staib F (1965) Serum-proteins as nitrogen source for yeast like fungi. Sabouraudia 4(3):187–193
Remold H, Fasold H, Staib F (1968) Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta 167(2):399–406
Naglik JR, Rodgers CA, Shirlaw PJ, Dobbie JL, Fernandes-Naglik LL, Greenspan D, Agabian N, Challacombe SJ (2002) Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis 188:469–479. https://doi.org/10.1086/376536
Jackson BE, Wilhelmus KR, Hube B (2007) The role of secreted aspartyl proteinases in Candida albicans keratitis. Invest Ophthalmol Vis Sci 48(8):3559–3565
Naglik J, Albrecht A, Bader O, Hube B (2004) Candida albicans proteinases and host/pathogen interactions. Cell Microbiol 6:915–926. https://doi.org/10.1111/j.1462-5822.2004.00439.x
Aoki W, Kitahara N, Miura N, Morisaka H, Yamamoto Y, Kuroda K, Ueda M (2011) Comprehensive characterization of secreted aspartic proteases encoded by a virulence gene family in Candida albicans. J Biochem 150:431–438. https://doi.org/10.1093/jb/mvr073
Navarathna DHMLP, Hornby JM, Hoerrmann N, Parkhurst AM, Duhamel GE, Nickerson KW (2005) Enhanced pathogenicity of Candida albicans pre-treated with subinhibitory concentrations of fluconazole in a mouse model of disseminated candidiasis. J Antimicrob Chemother 56:1156–1159. https://doi.org/10.1093/jac/dki383
Mann PA, McNicholas PM, Chau AS, Patel R, Mendrick C, Ullmann AJ, Cornely OA, Patino H, Black TA (2009) Impact of antifungal prophylaxis on colonization and azole susceptibility of Candida species. Antimicrob Agents Chemother 53:5026–5034. https://doi.org/10.1128/AAC.01031-09
Wu T, Wright K, Hurst SF, Morrison CJ (2000) Enhanced extracellular production of aspartyl proteinase, a virulence factor, by Candida albicans Isolates following Growth in subinhibitory concentrations of fluconazole. Antimicrob Agents Chemother 44:1200–1208. https://doi.org/10.1128/AAC.44.5.1200-1208.2000
Copping VMS, Barelle CJ, Hube B, Gow NAR, Brown AJP, Odds FC (2005) Exposure of Candida albicans to antifungal agents affects expression of SAP2 and SAP9 secreted proteinase genes. J Antimicrob Chemother 55:645–654. https://doi.org/10.1093/jac/dki088
Costa CR, Jesuíno RSA, Lemos JA, Fernandes OFL, e Souza LKH, Passos XS, Silva MRR (2010) Effects of antifungal agents in sap activity of Candida albicans isolates. Mycopathologia 169:91–98. https://doi.org/10.1007/s11046-009-9232
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeast. 4th ed. CLSI standard M27 (ISBN 1-46238-827-4). Pennsylvania, USA, 2017
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antifungal susceptibility testing of yeast. 1st ed. CLSI supplement M60 (ISBN 1-56238-829-0). Pennsylvania, USA, 2017
Kumar R, Shukla PK (2010) Amphotericin B resistance leads to enhanced proteinase and phospholipase activity and reduced germ tube formation in Candida albicans. Fungal Biol 114:189–197. https://doi.org/10.1016/j.fumbio.2009.12.003
Silva NC (2013) Aspartate protease (sap) analysis as a factor associated with the virulence of Candida albicans and Candida non-albicans strains. MD Thesis. Alfenas, Minas Gerais: Federal University of Alfenas; 2013
Bolano A, Stinchi S, Preziosi R (2001) Rapid methods to extract DNA and RNA from Cryptococcus neoformans. FEMS Yeast Res 1:221–224. https://doi.org/10.1111/j.1567-1364.2001.tb00037.x
Tavanti A, Pardini G, Campa D, Davini P, Lupetti A, Senesi S (2004) Differential expression of secretory aspartyl proteinase genes (SAP1-10) in Oral Candida albicans isolates with distinct karyotypes. J Clin Microbiol 42:4726–4734. https://doi.org/10.1128/JCM.42.10.4726-4734
Mandras N, Tullio V, Allizond V, Scalas D, Banche G, Roana J, Robbiano F, Fucale G, Malabaila A, Cuffini AM, Carlone N (2009) In Vitro activities of fluconazole and voriconazole against clinical isolates of Candida spp. determined by disk diffusion testing in turin. Italy. Antimicrob Agents Chemother 53:1657–1659. https://doi.org/10.1128/AAC.00998-08
Mnge P, Okeleye BI, Vasaikar SD, Apalata T (2017) Species distribution and antifungal susceptibility patterns of Candida isolates from a public tertiary teaching hospital in the Eastern Cape Province, South Africa. Braz J Med Biol Res 50:e5797. https://doi.org/10.1590/1414-431X20175797
Da Matta DA, Souza ACR, Colombo AL (2017) Revisiting species distribution and antifungal susceptibility of Candida bloodstream isolates from latin American Medical Centers. J Fungi 3:1–14. https://doi.org/10.3390/jof3020024
Braga-Silva LA, Santos ALS (2011) Aspartic protease inhibitors as potential anti-Candida albicans drugs: impacts on fungal biology, virulence and pathogenesis. Curr Med Chem 18:2401–2419. https://doi.org/10.2174/092986711795843182
Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence. 4(2):1–10. https://doi.org/10.4161/viru.22913
Fotedar R, Al-Hedaithy SSA (2005) Comparison of phospholipase and proteinase activity in Candida albicans and C. dubliniensis. Mycoses 48:62–67. https://doi.org/10.1111/j.1439-0507.2004.01057.x
D’Eça Júnior A, Silva AF, Rosa FC, Monteiro SG, Figueiredo PMS, Monteiro CA (2011) In vitro differential activity of phospholipases and acid proteinases of clinical isolates of Candida. Rev Soc Bras Med Trop 44:334–338. https://doi.org/10.1590/S0037-86822011005000036
Mattei AS, Alves SH, Severo CB, Guazzelli LS, Oliveira FM, Severo LC (2013) Determination of germ tube, phospholipase, and proteinase production by bloodstream isolates of Candida albicans. Rev Soc Bras Med Trop 46:340–342. https://doi.org/10.1590/0037-8682-0045-2013
Tellapragada C, Eshwara VK, Johar R, Shaw T, Malik N, Bhat PV, Kamath A, Mukhopadhyay C (2014) Antifungal susceptibility patterns, In Vitro production of virulence factors, and evaluation of diagnostic modalities for the speciation of pathogenic Candida from blood stream infections and vulvovaginal candidiasis. J Pathog. https://doi.org/1155/2014/142864
Jose NV, Mudhigeti N, Asir J, Chandrakesan SD (2015) Detection of virulence factors and phenotypic characterization of Candida isolates from clinical specimens. J Curr Res Sci Med 1:27–31
Hazen KC, Mandell G, Coleman E, Wu G (2000) Influence of fluconazole at subinhibitory concentrations on cell surface hydrophobicity and phagocytosis of Candida albicans. FEMS Microbiol Lett 183:89–94. https://doi.org/10.1111/j.1574-2000.tb08938.x
Ward M, Kodama KH (1991) Introduction to fungal proteinases and expression in fungal systems. Adv Exp Med Biol 306:149–160
Cannon RD, Fischer FJ, Niimi K, Niimi M, Arisawa M (1998) Drug pumping mechanisms in Candida albicans. Jpn J Med Mycol 39:73–78. https://doi.org/10.3314/jjmm.39.73
Graybill JR, Montalbo E, Kirkpatrick WR, Luther MF, Revankar SG, Patterson TF (1998) Fluconazole versus Candida albicans: a Complex Relationship. Antimicrob Agents Chemother 42:2938–2942. https://doi.org/10.1128/AAC.42.11.2938
Hube B, Ruchel R, Monod M, Sanglard D, Odds FC (1998) Functional aspects of secreted Candida proteinases. Adv Exp Med Biol 436:339–344
Naglik JR, Newport G, White TC, Fernandes-naglik LL, Greenspan JS, Greenspan D, Sweet SP, Challacombe SJ, Agabian N (1999) In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun 67(5):2482–2490
Bochenska O, Rapala-Kozik M, Wolak N, Aoki W, Ueda M, Kozik A (2016) The action of ten secreted aspartic proteases of pathogenic yeast Candida albicans on major human salivary antimicrobial peptide, histatin 5. Acta Biochim Pol 63(3):403–410
Hube B, Monod M, Schofield DA, Brown AJP, Gow NAR (1994) Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol 14:87–99. https://doi.org/10.1111/j.1365-2958.1994.tb01269
Felk A, Kretschmar M, Albrecht A, Schaller M, Beinhauer S, Nichterlein T, Sanglard D, Korting HC, Schäfer W, Hube B (2002) Candida albicans Hyphal Formation and the Expression of the Efg1-Regulated Proteinases Sap4 to Sap6 Are Required for the Invasion of Parenchymal Organs. Infect Immun 70:3689–3700. https://doi.org/10.1128/IAI.70.7.3689-3700.2002
Barelle CJ, Duncan VMS, Brown AJP, Gow NAR, Odds FC (2008) Azole antifungals induce up-regulation of SAP4, SAP5 and SAP6 secreted proteinase genes in filamentous Candida albicans cells in vitro and in vivo. J Antimicrob Chemother 61:315–322. https://doi.org/10.1093/jac/dkm456
Martínez P, Ljungdahl PO (2005) Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol Cell Biol 25:9435–9446. https://doi.org/10.1128/MCB.25.21.9435-9446.2005
Acknowledgements
The authors are thankful to Dr. Ivano de Filippis at the Laboratory of Reference Microorganisms of the National Institute of Health Quality Control (INCQS), Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, Brazil, for donating ATCC strains. We are also thankful to the laboratory of clinical analyses of the university hospital of UFBA for providing the clinical isolates. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Formal consent is not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
do Rosário Esteves Guimarães, C., de Freitas, H.F. & Barros, T.F. Upregulation of secreted aspartyl proteinase genes of fluconazole-sensitive Candida albicans isolates. Mol Biol Rep 46, 6147–6154 (2019). https://doi.org/10.1007/s11033-019-05049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05049-2