Abstract
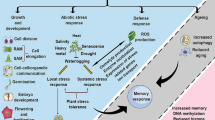
The expression of a gene encoding peroxisomal Cu-Zn superoxide dismutase from Saussurea involucrata Kar. et Kir. was induced by low temperature, PEG6000 treatment, and NaCl stress. To investigate the role of SikCuZnSOD3 in the mitigation of abiotic stress, we used Agrobacterium-mediated transformation to create transgenic cotton that overexpressed SikCuZnSOD3. Phenotypic analysis of T4 generation transgenic lines showed that they generally grew better than wild-type cotton under low temperature, PEG6000 treatment, and NaCl stress. Although there were no significant differences under control conditions, transgenic plants exhibited greater survival, fresh weight, and dry weight than wild-type plants under all three stress treatments. Additional physiological analyses demonstrated that the transgenic cotton had higher relative water content, proline and soluble sugar contents, and activity of antioxidant enzymes (superoxide dismutase, catalase, and peroxidase), as well as lower relative conductivity, malondialdehyde content, and H2O2 and O2− accumulation. More importantly, overexpression of SikCuZnSOD3 increased the yield of cotton fiber. Our results confirm that the overexpression of SikCuZnSOD3 can improve the abiotic stress resistance of cotton by increasing the activity of antioxidant enzymes, maintaining ROS homeostasis, and reducing cell membrane damage.







Similar content being viewed by others
Data availability
Datasets supporting the conclusions of this article are included within the article.
References
Abreu IA, Cabelli DE (2010) Superoxide dismutases—a review of the metal-associated mechanistic variations. Biochim Biophys Acta, Gen Subj 1804:263–274
Ahmad P, Umar S, Sharma S (2010) Mechanism of free radical scavenging and role of phytohormones in plants under abiotic stresses. Plant Adaptation and Phytoremediation 2010:99–118
Ahuja I, de Vos RC, Bones AM et al (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashraf M (2002) Salt tolerance of cotton: some new advances. Crit Rev Plant Sci 21:1–30
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Azarabadi S, Abdollahi H, Torabi M, Salehi Z, Nasiri J (2017) ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L.). Eur J Plant Pathol 147:279–294
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 1:205–207
Benikhlef L, L’Haridon F, Aboumansour E et al (2013) Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol 13:1–12
Bowler C, Slooten L, Vandenbranden S, de Rycke R, Botterman J, Sybesma C, van Montagu M, Inzé D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10:1723–1732
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Breusegem FV, Slooten L, Stassart JM et al (1999) Effects of overproduction of tobacco MnSOD in maize chloroplasts on foliar tolerance to cold and oxidative stress. J Exp Bot 50:71–78
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Ding FZ (2008) Research progress on application of superoxide dismutases in tobacco. J Anhui Agric Sci 36:1897–1894
Dong L, He YZ, Wang YL et al (2013) Research progress on application of superoxide dismutase (SOD). J Agric Sci Technol 15:53–58
Du ZY, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. Journal of Agricultural and Food Chemistry 40:1566–1570
Du XM, Yin WX, Zhao YX et al (2001) The production and scavenging of reactive oxygen species in plants. Sheng Wu Gong Cheng Xue Bao 17:121–125
Dupont CL, Neupane K, Shearer J et al (2010) Diversity, function and evolution of genes coding for putative Ni-containing superoxide dismutases. Environ Microbiol 10:1831–1843
Fan DY, Hope AB, Jia H, Chow WS (2008) Separation of light-induced linear, cyclic and stroma-sourced electron fluxes to P700+ in cucumber leaf discs after pre-illumination at a chilling temperature. Plant Cell Physiol 49:901–911
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Fukao T, Xu K, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18:2021–2034
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta, Gen Subj 990:87–92
Getzoff ED, Tainer JA, Stempien MM, Bell GI, Hallewell RA (1989) Evolution of CuZn superoxide dismutase and the Greek key beta-barrel structural motif. Proteins 5:322–336
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. 1. Occurrence in higher plants [corn, oats, peas]. Plant Physiol 59:309–314
Gil R, Lull C, Boscaiu M et al (2011) Soluble carbohydrates as osmolytes in several halophytes from a Mediterranean salt marsh. Not Bot Horti Agrobo 39:9–17
Greenway A, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Biol 31:61–69
Guo YY, Tian SS, Liu SS et al (2017) Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica 2017:1–12
Holmberg N, Bu Low L (1998) Improving stress tolerance in plants by gene transfer. Trends Plant Sci 3:61–66
Huang W, Zhang SB, Cao KF (2011) Cyclic electron flow plays an important role in photoprotection of tropical trees illuminated at temporal chilling temperature. Plant Cell Physiol 52:297–305
Jiang HF, Ren X (2004) The effect on sod activity and protein content in groundnut leaves by drought stress. Acta Agron Sin 3:1–4
Jiang Z, Zhu S, Ye R, Xue Y, Chen A, An L, Pei ZM (2013) Relationship between NaCl- and H2O2-induced cytosolic Ca2+ increases in response to stress in Arabidopsis. PLoS One 8:e76130
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218
Kurepa J, Smalle J, Van MM et al (1998) Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J Cell Mol Biol 14:759–764
Lara MV, Disante KB, Podesta FE et al (2003) Induction of a Crassulacean acid like metabolism in the C4 succulent plant, Portulaca oleracea L.: physiological and morphological changes are accompanied by specific modifications in phosphoenolpyruvate carboxylase. Photosynth Res 77:241–254
Leclercq J, Martin F, Sanier C, Clément-Vidal A, Fabre D, Oliver G, Lardet L, Ayar A, Peyramard M, Montoro P (2012) Overexpression of a cytosolic isoform of the HbCuZnSOD gene in Hevea brasiliensis changes its response to a water deficit. Plant Mol Biol 80:255–272
Lin QB, Ding YG, Wang HB et al (2009) The effects of the SOD mimics on the biomass and SOD activities of rice seedlings under salt stress. Chin J Soil Sci 40:1163–1166
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (T) (-delta delta C) method. Methods 25:402–408
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Miller G, Suzuki N, Ciftci-Yilmaz S et al (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Zilinskas BA (1994) Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J 5:397–405
Mittler R, Vanderauwera S, Gollery M, van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Moses JC (2012) Cu/Zn superoxide dismutase activity and respective gene expression during cold acclimation and freezing stress in barley cultivars. Biol Plant 56:693–698
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
Pham HM, Kebede H, Ritchie G, Trolinder N, Wright RJ (2018) Alternative oxidase (AOX) over-expression improves cell expansion and elongation in cotton seedling exposed to cool temperatures. Theor Appl Genet 131:2287–2298
Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA (1997) Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol 114:695–704
Pitzschke A, Forzani C, Hirt H (2006) Reactive oxygen species signaling in plants. Antioxid Redox Signal 8:1757–1764
Quan LJ, Zhang B, Shi WW et al (2010) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50:2–18
Razali N, Aziz AA, Lim CY et al (2015) Investigation into the effects of antioxidant-rich extract of Tamarindus indica leaf on antioxidant enzyme activities, oxidative stress and gene expression profiles in HepG2 cells. PeerJ 3:1227–1238
Song XS, Wang YJ, Mao WH, Shi K, Zhou YH, Nogués S, Yu JQ (2009) Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves. Physiol Plant 135:246–257
Sugimoto M, Oono Y, Gusev O, Matsumoto T, Yazawa T, Levinskikh MA, Sychev VN, Bingham GE, Wheeler R, Hummerick M (2014) Genome-wide expression analysis of reactive oxygen species gene network in mizuna plants grown in long-term spaceflight. BMC Plant Biol 14:4–4
Takemura S, Hanagata N, Sugihara K et al (2000) Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat Bot 68:15–28
Tepperman JM, Dunsmuir P (1990) Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol Biol 14:501–511
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions 160:1–40
Whittaker JW (2010) Metal uptake by manganese superoxide dismutase. BBA-Proteins and Proteomics 1804:298–307
Xia M, Wang W, Yuan R et al (2015) Superoxide dismutase and its research in plant stress-tolerance. Mol Plant Breed 13:2633–2646
Yan ZL, Niu JY, Xi LL et al (2009) Effect of soil water on protective enzyme activity and membrane lipid peroxidation in pea. Chin J Eco-Agric 17:554–559
Youn HD, Kim EJ, Roe JH et al (1996) A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochem J 318:889–896
Zeng XC, Liu ZG, Shi PH et al (2014) Cloning and expression analysis of copper and zinc superoxide dismutase (Cu/Zn-SOD) gene from Brassica campestris L. Acta Agronomica Sinica 40:636–643
Zhang Y, Tan J, Guo Z et al (2010) Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences. Plant Cell Environ 32:509–519
Zhang YT, Gao JM, Zhang QL et al (2016) Research progress on plant superoxide dismutase. AHFS 37:28–31
Zhu JB, Liu HL, Wang Z et al (2006) Construction of full-length cDNA library from the Saussurea involucrata Kar.et Kir Laminae. AABOS 15:170–173
Funding
This work was supported by the Xinjiang 2019 Postgraduate Scientific Research Innovation Project (Grant number XJ2019G084), Shihezi University–independently funded and supported project (Grant number ZZZC202082B), National GMO Major Project (Grant number 2016ZX080005004-009), and Key Scientific and Technological Projects for the Cultivation of New Varieties of Genetically Modified Organisms (Grant number 2018ZX0800501B-005).
Author information
Authors and Affiliations
Contributions
LZ: data curation and visualization; WHT: supervision and methodology; GH, major revision and formal analysis; BCL: software; AYW: writing (reviewing and editing); JBZ: validation and investigation; XYG: conceptualization and writing (original draft preparation). All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 7659 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Tian, W., Huang, G. et al. The SikCuZnSOD3 gene improves abiotic stress resistance in transgenic cotton. Mol Breeding 41, 26 (2021). https://doi.org/10.1007/s11032-021-01217-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-021-01217-0