Abstract
This study aimed to investigate the relationship between two aspects of emotional flexibility (EF, i.e. flexible emotional responsiveness and affective flexibility) and tolerance for, and recovery from, experimentally-induced pain. Fifty-two adults completed a flexible emotional responsiveness task in which emotional responses on multiple dimensions were registered while watching positive and negative pictures. Affective flexibility was measured using a switching task with positive and negative pictures. Pain tolerance was assessed as the time participants endured an ischemic pain task and total time until recovery in terms of pain intensity and pain unpleasantness was registered. Flexible emotional responsiveness—reflected in divergent corrugator responses to positive and negative stimuli—was associated with faster recovery from pain unpleasantness. Affective flexibility was associated with recovery in terms of pain intensity, with faster switches from neutral towards affective aspects of positive stimuli being predictive of faster recovery. Results did not provide evidence for an association between EF and pain tolerance. The findings suggest that EF may protect against pain persistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One of the characteristics of everyday life is that people have to adapt to constantly changing circumstances. As these changes naturally include alterations between positive and negative contexts (Sarason et al. 1978), successful adaptation requires flexibility at the emotional level (Bonanno et al. 2004). Emotional flexibility (EF) is defined as the ability to flexibly regulate emotions in a context-appropriate manner, and to recover from the primary emotional response when the context changes, thereby creating a best possible match with the ever changing environment (Aldao et al. 2015; Beshai et al. 2018; Westphal et al. 2010). EF also refers to the flexible use of different emotion regulation strategies that fit the situation at hand, and is adaptive when this flexibility increases the chances of attaining personal relevant goals (Aldao et al. 2015). Hence, EF is essential for adequate emotion regulation.
Several studies have identified a link between EF and resilience, here defined as the ability to effectively adapt to both major and every day’s life events (Block and Kremen 1996). Waugh et al. (2011) demonstrated that high resilient participants showed more distinct emotions and facial expressions in response to alternating positive and negative stimuli and were thus better capable to switch their emotional responses in order to emotionally adapt to these stimuli. Likewise, more flexibility in switching between emotional contexts has been related to trait resilience (Genet and Siemer 2011), more use of adaptive emotion regulation strategies such as reappraisal (Malooly et al. 2013), and less rumination (Genet et al. 2013).
EF may also affect how an individual copes with pain and as such, may function as a candidate mechanism in explaining individual differences in pain tolerance and recovery. Besides a sensory experience, pain is also considered to be an emotional experience (e.g., Craig 2003). Given this emotional dimension of pain, coping with pain requires controlling your emotions or regulating your emotional responses to painful stimuli. Indeed, a large number of studies has consistently pointed out a role for emotion regulation in pain experience, thereby indicating that difficulties in emotion regulation are associated with higher distress and pain levels (e.g. Keefe et al. 2001). For instance, maladaptive emotion regulation is directly associated with pain-related disability, depressive symptoms, and pain chronicity (as systematically reviewed in Koechlin et al. 2018). EF in the context of pain refers to the capability to respond emotionally to painful events, and recover from these emotional responses when the pain episode is over. Along this line of reasoning, EF may act as a mechanism explaining why some individuals can better tolerate pain and recover faster from pain.
Experimental research generally focuses on two aspects of EF, which both are believed to facilitate effective emotion regulation (Gross 2007). The first aspect, flexible emotional responsiveness, is the ability to flexibly produce emotional responses to alternating positive and negative circumstances (Hollenstein et al. 2013; Westphal et al. 2010). Hence, positive responses should harmonize with positive events and negative responses with negative events (Waugh et al. 2011). Identifying pain and responding effectively with negative emotions (i.e. flexible emotional responding) serves an adaptive function, as it results in the activation of protective behavior including avoidance, escape, and social alarm (Eccleston and Crombez 1999; Sullivan 2008). Flexible emotional responsiveness may therefore be essential for managing pain, as the function of acute pain is to signal potential physical harm or illness (Eccleston and Crombez 1999).
The second component of EF is affective flexibility, a specific kind of cognitive flexibility, and refers to the capacity to switch between emotional information and neutral aspects of the environment (Genet et al. 2013; Genet and Siemer 2011; Malooly et al. 2013). In pain research, ample evidence exists for the negative effect of acute and chronic pain on cognitive flexibility, including shifting and inhibition performance (e.g. Boselie et al. 2014; Karp et al. 2006; Van Ryckeghem et al. 2012; Verdejo-Garcia et al. 2009). Furthermore, cognitive flexibility has been suggested to influence the experience of pain (Bjekić et al. 2018; Karsdorp et al. 2014; Oosterman et al. 2010) and even the development of chronic pain after surgery (Attal et al. 2014). Given these findings and the emotional characteristics of pain, it is reasonable to suggest that cognitive flexibility specific to emotional material (i.e. affective flexibility) plays a potentially important role in pain sensitivity. More precisely, affective flexibility can be proposed to function as an explanatory mechanism in how people manage and recover from their pain.
This study aims to provide a first test of the proposed relation between EF and pain by examining the associations between EF (i.e. flexible emotional responsiveness and affective flexibility) and pain sensitivity. To this purpose, an experimental pain induction procedure is used and both pain tolerance and recovery in terms of pain intensity and pain unpleasantness are assessed. EF is measured by means of two computer tasks that tap into flexible emotional responsiveness and affective flexibility, respectively. It is hypothesized that more EF is related to higher pain tolerance and a quicker recovery from both pain intensity and pain unpleasantness. Current findings will contribute to our knowledge on the potential of EF to explain individual differences in pain experience and recovery from pain.
Methods
Participants
Participants were 56 healthy adults recruited to participate in a cross-sectional observational study through advertisements at Maastricht University and via an electronic research participation system (SONA). Exclusion criteria were pregnancy, a diagnosis of a psychiatric or neurological disorder, cardiovascular disease, injury or pain to the non-dominant arm, acute or chronic pain, blood pressure problems (hypertension/hypotension), the use of anticoagulants or medication with this feature, blood clotting problems, severe medical diseases (e.g. diabetes, asthma), the use of anxiolytics or antidepressants, and carrying an electronic implant. These criteria were all checked by means of self-report prior to scheduling a test appointment and at the moment the participants showed up for their test appointment at the lab. Four participants were not eligible and excluded at the start of the lab session because of non-compliance. The final sample consisted of 52 subjects (mean age 22.7 years; SD = 3.81; range 18–39 years; 8 male). The pain induction had to be interrupted prematurely for two participants due to lack of time for the entire experiment, making the tolerance and pain intensity and pain unpleasantness recovery data for these participants invalid. The participants received either a 20-euro gift voucher or course credits as incentive. The study protocol received approval by the ethical review committee of the Faculty of Psychology and Neuroscience (ERCPN), Maastricht University.
Measures
Pain experience and recovery from pain
Handgrip strength
A Bremshey Sport® electronic hand dynamometer (Almere, The Netherlands) was utilized to measure maximum handgrip strength of the non-dominant hand in kg/lb. The mean across two maximum handgrips was calculated.
Submaximum effort tourniquet technique
The submaximum effort tourniquet technique (SETT) was used to induce ischemic pain (Smith et al. 1966), a type of pain caused by restriction of blood supply to the tissue. This pain method produces a tonic deep aching pain which is more comparable to pain of pathologic origin in terms of severity and duration than most other pain stimuli (Moore et al. 1979). In comparison to other available experimental pain induction methods (e.g. the cold pressor task or thermal stimulation), the SETT typically takes longer time to fully recover from, making it a suitable method to examine (individual) differences in recovery from pain in a lab situation. Blood pressure was first determined with an automatic Omron M3 Intellisense blood pressure monitor (OMRON Corporation, Kyoto, Japan) applied to the non-dominant upper arm. Blood pressure was measured at the start of the experiment in order to verify that participants did not have hypertension (i.e. systolic blood pressure > 133 mmHg) and again prior to the start of the SETT. In case these two measurements deviated more than 10 mmHg, blood pressure was measured for a third time. Mean systolic blood pressure was calculated across the two measurements or in case of three measurements across the two values that were closest to one another. During the SETT, an aneroid Minimus® II sphygmomanometer cuff (Riester, GmbH, Jungingen, Germany) was attached to the non-dominant upper arm. The participant was requested to reach the arm towards the ceiling for 1 min in order to drain blood from the arm. The arm was then put to rest on the table and the cuff was inflated to 1.5 × the mean systolic blood pressure to obstruct blood flow. After 1 min, the participant squeezed a hand trainer 12 times for 2 s with a 2-s break in between squeezes. A digital metronome indicated the pace of these squeezes. Afterwards, the subject was asked to put the arm to rest again on the table.
Pain intensity and pain unpleasantness
Oral numerical rating scales (NRS) were employed for both pain intensity (0 = ‘not painful at all’; 10 = ‘unbearably painful’) and pain unpleasantness (0 = ‘not unpleasant at all’; 10 = ‘extremely unpleasant’) ratings during the SETT and recovery period. The timing of these ratings is described in more detail in the “Procedure” section. The time from the last squeeze with the hand trainer to SETT termination was taken as measure for pain tolerance (s). Time to total recovery in terms of pain intensity (‘pain intensity recovery’) and pain unpleasantness (‘pain unpleasantness recovery’) was assessed as the time (s) from cuff deflation till the moment the participant gave a pain intensity or a pain unpleasantness rating of 0, respectively. For exploratory purposes, a few additional written visual analogue scale (VAS) scores were obtained before pain induction and at session completion, but these were not further analyzed.
Emotional responsiveness task
Task set-up
A slightly adjusted version of the task designed by Waugh et al. (2011) was utilized to measure flexible emotional responsiveness to alternating positive and negative pictures. Emotional responsiveness was continuously assessed across multiple modalities, namely self-reported affect, facial expressions, and defensive motivation as indexed by eye blink startle amplitude.
In short, each trial starts with the presentation of a cue (i.e. ‘+’ or ‘−’), indicating whether the trial will be positive or negative, respectively. Trials consist of three pictures of the same valence that are presented consecutively for 4 s each against a black background. Afterwards, the participant views a black screen for 2 s. Pictures are taken from the International Affective Picture System (IAPS; Lang et al. 1997). Mean arousal values for the IAPS pictures as based on IAPS development studies do not differ significantly across trial type: positive = 5.41, negative = 5.38, t(78) = − 0.33, p > .05. Similarly, there are no differences in mean picture valence across trial type when positive images are reverse-scored: positive = 2.63, negative = 2.57, t(78) = − 0.48, p > .05.
The task is divided into two blocks of 41 trials each, making a total of 82 trials with 246 pictures. The blocks are separated by a 1-min break. Each block consists of a fixed order stimulus list for the 41 trials. The stimulus lists are created in a pseudo-random manner, thereby ensuring equal trial type distribution, no more than three successive presentations of the same trial type, and a positive first trial in each block. Emotional responses to the first trial of each block are eliminated from analyses. On a random 24 out of 40 trials per trial type (60% of the trials, disregarding the first trial of each block) an acoustic startle probe (50 ms) is administered binaurally through headphones. Startle probes are 95 dB(A) bursts of white noise with instantaneous rise time administered during the presentation of the second picture (1.5 s after picture onset).
Self-reported affect
A rating dial is used to assess self-reported affect continuously throughout the emotional responsiveness task. The dial used in this study was custom-made by the instrumentation engineering department of the Faculty of Psychology and Neuroscience, Maastricht University and consists of a 44-mm diameter black dial with a triangle-shaped white pointer depicted on it that revolves 180°. In addition to the original task developed by Waugh et al. (2011), our task had an affective scale consisting of a grey 180° semicircle and a white pointer that was continuously presented below the stimuli on the screen throughout the entire task. This scale was added to make sure that participants are aware of the exact location of the pointer without having to switch their gaze between the screen and the dial. The anchor 0 (‘negative’) is positioned at 0° and the anchor 9 (‘positive’) at 180°, resulting in a neutral rating of 4.5 at 90°, yet not explicitly indicated. Only data for the first picture of each trial are used and thus registered at 4 s after onset of the first picture of each series (Waugh et al. 2011). This moment was chosen because it minimizes the effect of the previous position of the dial on current ratings (Waugh et al. 2011). Furthermore, it is expected that affective responses have stabilized after this time. Scores were recoded by subtracting 4.5 in order to let negative scores indicate negative self-reported affect and positive scores indicate positive affect. Next, average affect was calculated per trial type. Lastly, a difference score between the affective ratings to positive and negative pictures was computed. This new variable was named DiffAffect. Due to technical difficulties, affect ratings for one participant had to be excluded from subsequent analyses.
EMG recordings
Facial expressions are measured in terms of muscle activity using facial electromyography (EMG) over two facial muscles: the zygomatic major and the corrugator supercilii muscles (Fridlund and Cacioppo 1986). Zygomatic activity is recorded as an index for positive emotions, whereas activation of the corrugator is an index for negative emotions (Cacioppo et al. 1986; Tassinary and Cacioppo 1992). Mean zygomatic and corrugator responses to the first picture are used for analyses.
In addition, acoustically elicited startle reflexes during the presentation of positive and negative pictures are assessed to measure defensive motivation, which involves the increase of aversive responses (startle response) in an unpleasant context (Lang et al. 1990). Specific to this task, startle reflexes are augmented when viewing negative pictures and diminished when viewing positive pictures. Startle reflexes are recorded over the orbicularis oculi in response to the second picture in order to prevent confounding of self-report and EMG responses to the first picture of a sequence with the administration of a startle probe.
Physiological data were recorded with a BrainAmp ExG® amplifier (Brain Products GmbH, Gilching, Germany). In line with recommendations made by Fridlund and Cacioppo (1986), three pairs of 4-mm Ag/AgCl surface electrodes were positioned over the zygomatic major, corrugator supercilii, and the orbicularis oculi muscles. A seventh 4-mm reference electrode was placed behind the ear on the mastoid part of the temporal bone. Electrodes were disc shaped, had a diameter of 2 mm with a gel bed of 4 mm in an external diameter of 11 mm. Before electrode placement, participants were asked to clean their face with cleansing milk and the skin was subsequently cleaned with 70% alcohol solution and gently scrubbed with skin prep gel (Nuprep®, Weaver and Company, CO, USA). Electrodes were filled with a conductive EMG paste (Ten20®, Weaver and Company, CO, USA) and attached to the skin surface using double-sided adhesive collars. Inter-electrode distance between electrode centers was approximately 10 mm. Electrodes were placed on the side of the face that was opposite to the dominant side of the body. For three participants, the electrodes were placed on the right side of the face because of left handedness. Before the recordings started, impedance was checked to ideally be between 0 and 5 kΩ and to not exceed 20 kΩ (Tassinary et al. 2007). The raw EMG data was sampled at 1 kHz and bandpass filtered (10- to 1000-Hz) with the BrainVision Recorder software. BrainVision Analyzer software (version 2.0) was used to process the EMG and startle data offline.
EMG preprocessing and data reduction
-
Corrugator and zygomatic activity Raw EMG data were visually inspected for artefacts (i.e. noise, movement artefacts). Of the 4000 measurement points, 346 (8.7%) were rejected for the corrugator channel, and 221 (5.5%) for the zygomatic channel. Following offline filtering (28 Hz high-pass filter, 48 dB/octave), EMG data were rectified and smoothed (50-ms moving window average). The epoch that was analyzed for corrugator and zygomatic muscle activity covered the total duration of the first picture of each series (4 s). For each muscle, mean EMG activity was baseline corrected by subtracting the average EMG activity during the last 2 min of a 5-min baseline measurement from the average corrugator and zygomatic activity during the first picture on each trial and expressed in µV. Data for two participants were excluded from further analyses, as no reliable baseline measurements were obtained. Following Waugh et al. (2011), outlier identification was done on the basis of within subject means and SD and were defined as being above or below 3 SD from the mean. Outliers were then replaced by the value corresponding to 3 SD above or below the mean. Next, mean zygomatic and corrugator activity per trial type was calculated. A Matlab script was developed in order to perform all data processing steps (Matlab 7.17, MathWorks Inc., USA).
-
Eye blink startle responses After data acquisition, raw EMG signals were visually inspected for artefacts (i.e. noise, movement artefacts, and double blinks − 50 to 150 ms relative to startle probe onset). From the 2496 measurement points, 527 (21.1%) contained artifacts and were excluded. After offline filtering (100 Hz low-pass, 48 dB/octave), data were rectified and smoothed (20-ms time moving window average). The recommendations made by Tassinary et al. (2007) were followed for the scoring of startle responses. The window for onset latency for the startle reflex was set on 50–150 ms after probe onset. Peak amplitude was determined within this window and scored as a startle reflex when it was larger than 5 SD above the peak amplitude of a pre-stimulus window of 50 ms duration. Response amplitude (µV) was obtained by subtracting the average EMG value during the pre-stimulus period from the value at peak amplitude. Trials that were identified as nonresponse trials were rejected, which was the case for 19.9% of the trials. Considering artefact and nonresponse eyeblink rejection, 66.7% of the trials remained for further analyses. After standardization of the response amplitudes with within subject T score transformations, outliers were corrected by substituting responses smaller or larger than 3 SD from the mean with the value corresponding to 3 SD above or below the mean (Waugh et al. 2011). Outlier identification was done on the basis of within subject means and SD.
Scoring of EMG data
We first checked whether positive trials were rated more positively, elicited higher zygomatic activity, lower corrugator activity, and lower startle amplitudes than negative trials using paired samples t tests. Next, a difference score between the facial muscle responses and startle amplitude to positive and negative pictures was calculated, resulting in the following three variables: DiffZyg (zygomatic response to positive pictures–zygomatic response to negative pictures), DiffCor (corrugator response to negative pictures–corrugator response to positive pictures), and DiffStartle (startle response to negative pictures–startle response to positive pictures). Higher difference scores reflect greater responses that are congruent to picture valence (e.g., higher corrugator activity in response to negative pictures) and thus more flexibility in emotional responsiveness.
Affective switching task
Task set-up
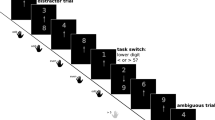
The affective task switching paradigm designed by Genet et al. (2013) was used to measure affective flexibility. In short, participants view emotionally valenced pictures on a computer screen and categorize these according to either an affective rule (determining whether the picture is positive or negative) or a neutral rule (determining whether the picture contains one or none humans, or two or more humans). Reaction times (RTs) are recorded in this task. The stimuli are 160 pictures from the IAPS (Lang et al. 1997) that can be classified into four categories based on the two sorting rules. Pictures are displayed one by one in the center of a monitor with the active categorization rule (the cue) depicted on the left and right side of the picture; ‘+’ and ‘−’ indicate positive and negative, ‘≤ 1’ and ‘≥ 2’ indicate one or none people and two or more people. The picture is displayed against either a white or grey background with the color of the background corresponding to the cue. Pictures are shown until a response is made by pressing one of two keys on a response box; the left-hand index finger was on the left button and the right-hand index finger on the right button. The picture category and cue are combined according to a pseudo-random order. There are eight task versions based on the mapping of the frame color (white or grey) to the active categorization rule (valence or number of people), and mapping of the response keys (left or right) to the categorization rule (‘+’/‘−’ or ‘≤ 1’/‘≥ 2’).
The task consist of two 10-trial practice blocks in which one of two rules is practiced per block and two 160-trial test blocks in which both rules are presented. Trials can either be a switch (trials on which the categorization rule changes) or a repeat trial (trials on which the same rule is repeated). It is important to note that the valence of the pictures never changes in the switch trials; it is only the categorization rule that alternates. Similar numbers of switches and repeats were made in all four stimulus conditions. Switch costs are calculated by subtracting RTs on repeat trials from RTs on switch trials. Lower switch costs (i.e. faster responses to switch tasks) reflect higher affective flexibility.
Preprocessing and scoring of task switching data
Incorrect responses were obtained for 7.4% of the trials and excluded from subsequent analyses. Following previous research using the same task (Genet et al. 2013; Grol and De Raedt 2017; Malooly et al. 2013), the impact of outliers was reduced for 0.006% of the trials by replacing RTs equal to or smaller than 250 ms with the value of 250 ms. In addition, individual outlying values, identified as RTs larger than 2.5 SD above the individual mean, were set equal to individual mean plus 2.5 SD (2.95% of the trials).
Based on both the active categorization rule and picture valence of the current and previous trials, mean RTs for eight trial types were computed: four repeat trial types (positive to positive rule, negative to negative rule, neutral to neutral rule for positive pictures, neutral to neutral rule for negative pictures), and four switch trial types (positive to neutral rule, negative to neutral rule, neutral to positive rule, neutral to negative rule).
Four types of switch costs were computed by subtracting RTs on repeat trials from RTs on switch trials. More specifically, switch costs for affective-to-neutral (AtoN) task sets were computed by subtracting RTs on affective repeat trials from RTs on affective-to-neutral trials: switch cost AtoN = switch (affective to neutral) − repeat (affective to affective). This type of switch cost was separately computed for positive (SC posAtoN) and negative (SC negAtoN) pictures. Similarly, the following formula was employed to calculate switch costs for neutral-to-affective (NtoA) task sets for positive (SC posNtoA) and negative (SC negNtoA) pictures separately: SC NtoA = switch (neutral to affective) − repeat (neutral to neutral).
Questionnaires
Psychological questionnaires were administered for exploratory purposes (i.e. exploring the relationship between the EF measures and psychological personality constructs, such as general resilience and fear constructs) and are not further reported in the current paper. These included the 9-item Acceptance and Action Questionnaire-II (AAQ-II; Bond et al. 2011; Fledderus et al. 2012), the Life Orientation Test-Revised (LOT-R, Scheier et al. 1994), the 10-item Connor-Davidson Resilience Scale (CD-RISC-10; Campbell-Sills and Stein 2007), the Highly Sensitive Person (HSP) Scale (Aron and Aron 1997), the Pain Catastrophizing Scale (Sullivan et al. 1995), the Fear of Pain Questionnaire-Short Form (Asmundson et al. 2008), the State-Trait Anxiety Inventory Y2 form (Spielberger et al. 1983) and four questions concerning stress levels.
Procedure
Recruitment and testing of participants took place from March until April 2016 at Maastricht University. On arrival to the lab, study eligibility was checked and written informed consent was obtained from each participant. The study could be completed in either Dutch or English; 34 (65.38%) participants chose English as language. Participants’ non-dominant hand and arm were checked for wounds. A disruption of the skin was classified as a (healing) wound for which the skin integrity is/was broken and there is/was a hemorrhage. It was important to check for wounds, as occlusion of the blood flow during the SETT may cause new bleedings. No participant had to be excluded because of this criterion. The experimenter then performed the first blood pressure measurement. Participants were instructed not to speak and to relax during this measurement. Next, maximum handgrip strength was assessed by asking the participants to squeeze the hand dynamometer twice as strongly as possible. Basic sociodemographic information (age, gender, mother tongue, nationality, type and year of study) was then obtained and participants completed the psychological questionnaires on the computer using an online survey application (Qualtrics, Provo, UT).
After the experimenter had attached the facial electrodes, baseline EMG activity was measured during a 5-min resting period. In addition, a Polar® RS800CX (Polar CIC, USA) heart rate band was attached around the chest in order to assess heart rate variability. However, due to too many artifacts in the recordings, these data could not be used. Next, participants completed the emotional responsiveness task during which continuous facial EMG data were recorded. Participants rated their current feelings throughout the task (i.e., during the viewing of the cues, the pictures and the inter stimulus intervals) using the rating dial. Participants were explicitly instructed that they could turn the dial as frequently as their feelings altered throughout the task. They were also informed that they would hear loud sounds at random points throughout the task, but that they should try not to pay any attention to these sounds. The task started with the presentation of four example pictures (two positive and two negative pictures) and five example startle probes, followed by 82 trials with 246 pictures.
After the emotional responsiveness task, participants completed the affective switching task in which they categorized pictures according to the affective and non-affective rule. The eight versions of the task were counterbalanced across participants. The emotional responsiveness task always preceded the affective switching task. This way, the assessment of initial emotional responses in the emotional responsiveness task was not affected by prior exposure to similar pictures in the affective switching task.
After task completion, the experimenter took a second blood pressure measurement and explained the procedure for the SETT. Participants were told that it would be completely normal if their arm would turn white or become cold or numb during the SETT and that this would almost immediately disappear upon cuff deflation. Participants were not informed about the duration of the SETT: the task was ended by the experimenter when 20 min had passed, a maximum pain intensity score of 10 was given, or when the participant expressed the wish to stop. Participants were made familiar with the sound and pace of the digital metronome that indicated the pace at which the hand trainer had to be squeezed and were then allowed to practice. During the SETT, participants were asked to verbally rate how painful and unpleasant the sensations in their entire arm felt. These two types of ratings were administered in immediate succession at 16 time points, namely at 0, 1, 2.5, 4.5, 5.5, 7.5, 9, 10.5, 11.5, 13.5, 15, 16, 17.5, 18, 19, and 20 min after completion of the last squeeze with the hand trainer. Less than 16 ratings were recorded in case earlier SETT termination took place due to a maximum pain intensity rating of 10 or the participant’s wish to stop. In addition, oral NRSs for pain intensity and pain unpleasantness were administered at 0, 45, 105, 150, 205, 275, 335, 385, 430, 475, 535, and 600 s (10 min) after cuff deflation until both pain intensity and pain unpleasantness ratings of 0 were given.
After participants had completed the SETT, they filled out an exit questionnaire that included questions concerning experiences during the SETT, the goal of the study, and the two switching tasks (attention and motivation to perform the tasks, pleasantness, interest in the task, task duration and difficulty). Upon leaving the lab, participants were queried for lingering sensations caused by the SETT. If so, participants would receive a phone call by the experimenter the same evening as to verify whether these sensations had worn off, which was the case for two participants. The experiment lasted approximately 2 h. Participants received a written debriefing via e-mail after the last participant had been tested. This experiment was part of a larger study in which diary data was also collected after the lab session. Results from these diary measurements will be described elsewhere.
Statistical analyses
The sample size was estimated using G*power (version 3.1.9.2) with hierarchical multiple regression analyses as our main analyses. A minimal sample size of 50 participants was required when a medium effect size (f = 0.15), a power of 0.85, and a significance level of .05 were taken into account (one-tailed). Statistical analyses were conducted using SPSS version 24 software (SPSS, Inc, IL, USA). Descriptive statistics were computed and data were checked to follow Gaussian distributions. Homogeneity of variance was checked and outliers were defined as being 3 SD below or above the mean and replaced by this value for questionnaire and pain-related variables. Our outcome measures were pain tolerance (s), pain intensity recovery (s), and pain unpleasantness recovery (s) and were analyzed by using multiple hierarchical regression analyses. Significance levels were set at .05.
Two sets of hierarchical multiple regression analyses were performed with each set testing the predictive value of variables derived from either the emotional responsiveness task or the affective switching task for each of the pain-related outcome variables (i.e. pain tolerance, pain intensity recovery, and pain unpleasantness recovery). The first set of regression analyses tested the predictive value of the parameters of the emotional responsiveness task (i.e. DiffAffect, DiffCor, DiffZyg, and DiffStartle) with the three pain-related outcomes. The second tested the predictive value of the parameters of the affective switching task (i.e. SC posAtoN, SC negAtoN, SC posNtoA, and SC negNtoA) with the three pain-related outcomes. In all regression models, age, sex and maximum handgrip strength were entered at the first step and the different EF parameters at the second step. For the analyses of pain intensity and pain unpleasantness recovery, we also controlled for pain tolerance by adding this variable in the first step of the model. Age, sex and maximum hand grip strength did not significantly predict any of the outcome variables (β [− 0.27, 0.14], all p’s > .071) and were thus removed from all the regression models. In addition, a backward deletion method was also used for both sets of analyses. For significant models, both the full and the final model after deletion of the non-significant predictors are shown.
Results
Pain tolerance and recovery scores
Fifteen (30%) of the 50 participants did not terminate the SETT before 20 min had passed, for whom a pain tolerance score of 1200 s was registered. In the present sample, a mean pain tolerance of 762.26 s (SD = 395.61), a mean pain intensity recovery of 254.00 s (SD = 152.14) and a mean pain unpleasantness recovery of 369.00 s (SD = 157.61) was obtained.
Emotional responsiveness task
Paired samples t tests were performed to test whether responses to positive trials differed from responses to negative trials in terms of affect ratings, corrugator and zygomatic activity, and startle blinks. For self-reported affect, positive trials were not rated more positively (M = − 0.47, SD = 0.90) than negative trials (M = − 0.46, SD = 0.74), t(51) = 0.095, p = .93. For the corrugator muscle, the baseline corrected activity in response to negative trials (M = 0.12, SD = 0.13) was, as predicted, higher than in response to positive trials (M = − 0.12, SD = 0.12), t(49) = − 6.78, p < .001, d = 1.94. In addition, positive trials elicited higher baseline corrected zygomatic activity (M = 0.091, SD = 0.12) than negative trials (M = − 0.093, SD = 0.12), t(49) = 5.24, p < .001, d = 1.50. As expected, startle amplitudes were larger on negative trials (M = 50.91, SD = 1.94) than on positive trials (M = 49.12, SD = 2.18), t(49) = − 3.24, p = .002, d = 0.93.
Table 1 shows the mean and SD of the difference between responses to positive versus negative trials for the emotional responsiveness task variables as well as their inter-correlations. Greater differential corrugator responses to negative versus positive pictures were related to greater differential zygomatic responses.
Flexible emotional responsiveness as predictor for pain tolerance and recovery
We decided to not include DiffAffect as a predictor in the regression analyses, as no significant difference was found between responses on positive and negative trials.Footnote 1 Variance inflation factors (VIFs) were checked for each model (range 1.00–1.73) and did not suggest any problems concerning collinearity between predictors. For pain tolerance and pain intensity recovery as dependent variables, no significant regression models were identified (pain tolerance: R2 = .054, F(3, 44) = 0.84, p = .48; pain intensity recovery: R2 = .19, F(4, 43) = 2.56, p = .052). For pain unpleasantness recovery, the regression model reached significance (R2 = .52, F(4, 43) = 11.81, p < .001), with only pain tolerance as a step 1 significant predictor (pain tolerance: β = 0.67, p < .001; DiffCor: β = − 0.21, p = .087; DiffZyg: β = − 0.066, p = .59, DiffStartle: β = 0.094, p = .39). After backward deletion of step 2 insignificant predictors, DiffCor resulted as an additional significant predictor (R2 = .51, F(2, 45) = 23.76, p < .001; DiffCor: β = − 0.25, p = .020; pain tolerance: β = 0.67, p < .001). Hence, a greater differential response in terms of corrugator activity was associated with faster recovery in terms of pain unpleasantness.
Affective switching task
A paired samples t test revealed that in general, RTs on switch trials (M = 1321.18 ms, SD = 199.45 ms) were significantly larger than RTs on repeat trials (M = 1168.52 ms, SD = 166.81 ms), t(51) = − 14.88, p < .001, d = 4.17.Footnote 2 Table 2 shows the mean and SD of the different types of switch costs as well as their correlations. In general, switch costs were highest when a switch from the affective to non-affective rule was required. The different types of switch costs correlated weak to moderately with one another, illustrating that these switch costs reflect distinct components of affective flexibility.
Affective flexibility as predictor for pain tolerance and recovery
For each regression model, VIFs (range 1.00–1.76) indicated no problems of collinearity between predictors. For pain tolerance, no significant regression model was identified (R2 = .076, F(4, 45) = 0.93, p = .46). Table 3 shows the results from hierarchical multiple regression analyses for pain intensity and pain unpleasantness recovery. For pain intensity recovery, longer recovery times were significantly predicted by higher costs resulting from switches toward the affective rule when viewing positive pictures (SC posNtoA) (R2 = .28, F(5, 44) = 3.47, p = .010). Similar results were found when performing the backward procedure (R2 = .24, F(2, 47) = 7.27, p = .002). For pain unpleasantness recovery, the regression model reached significance (R2 = .54, F(5, 44) = 10.21, p < .001), with only pain tolerance as a significant and SC posNtoA as a borderline significant predictor (pain tolerance: β = 0.63, p < .001; SC posAtoN: β = − 0.034, p = .76; SC negAtoN: β = 0.17, p = .15; SC posNtoA: β = 0.21, p = .053; SC negNtoA: β = − 0.056, p = .62). Backward deletion leaded to similar results (R2 = .46, F(1, 48) = 41,32, p < .001).
Discussion
The current study investigated the relationship between EF (i.e. flexible emotional responsiveness and affective flexibility) and tolerance for, and recovery from experimentally-induced ischemic pain. Two tasks were utilized to tap into these different aspects of EF, namely an emotional responsiveness task in which self-report affect and facial EMG were continuously registered, and an affective switching task. Ischemic pain was induced by using a Tourniquet pain procedure, which enabled us to measure both pain tolerance and recovery from pain. Neither EF measure was found related to pain tolerance. More flexible emotional responding in terms of corrugator muscle activity was associated with faster recovery from pain in terms of pain unpleasantness. Furthermore, affective flexibility was negatively related to recovery from pain in terms of pain intensity. This was especially the case when a shift from evaluating neutral aspects towards emotional aspects of positive stimuli was required. Taken together, these findings suggest that EF contributes to the recovery from pain.
The emotional responsiveness task was used as an index of the ability to flexibly generate emotional responses to changing stimuli. In general, distinct responses to alternating events with a relatively fast recovery afterwards are considered adaptive and an important characteristic of EF (e.g. Papousek et al. 2012). In contrast, prolonged but weak responses are believed to be maladaptive and a sign of inflexibility. For instance, depressive symptoms are related to reduced positive and negative emotional responses (Bylsma et al. 2008) and impaired recovery from an emotional event (Chida and Hamer 2008). Greater differential responses to positive versus negative pictures across different modalities in our task are considered to reflect the distinct short-lived responses characteristic for EF.
Along the lines of our hypotheses, greater differential responses to negative compared to positive pictures expressed in corrugator muscle activity were related to faster recovery from pain unpleasantness. More divergent corrugator activity to negative versus positive stimuli reflects the capacity to adequately discriminate between negative and positive stimuli and respond accordingly (Waugh et al. 2011). This divergent corrugator response to negative compared to positive stimuli has already been associated to higher trait resilience (Waugh et al. 2011). Here, we found that it also predicts resilient responding in the face of pain, i.e. faster recovery from pain unpleasantness.
Differential zygomatic major and startle blink responses were not associated with pain tolerance or recovery. The corrugator muscle is more sensitive to valence than the zygomatic major (Larsen et al. 2003), which could explain why we only found an effect for the corrugator channel. In the same manner, differential startle responses to positive compared to negative pictures found in this study could have been too small to be predictive of pain outcomes. However, future research is needed to further elucidate the role of flexible emotional responsiveness across different response modalities in pain experience and recovery from pain.
Affective flexibility was assessed using an affective switching task. In this study faster switching from processing neutral towards affective aspects of positive pictures was related to faster recovery of pain intensity. Previously, it was shown that people who were faster in performing these specific type of switches were also more likely to use reappraisal for the down-regulation of affective responses to a sad movie fragment (Malooly et al. 2013). Hence, people who more easily direct themselves to the positive elements of their environment may apply adaptive emotion regulation strategies such as reappraisal to recover from an affective challenge like pain. In other words, more flexibility towards positive aspects of positive pictures can be considered adaptive.
Some possible limitations of the present study should be noted. First, participants did complete the EF tasks in anticipation of a potentially unpleasant/painful task, which might have interfered with task performance (e.g. Van Damme et al. 2004). Future research may counter this problem by administering the EF tasks and the painful task on separate test occasions. Second, our data did not reveal differential self-report affective responses to positive and negative stimuli. One explanation could be that participants did not adhere very well to the instruction to provide ratings for each picture shown. On average, participants only turned the rating dial on 14.13% of all trials. Furthermore, they reported to have experienced the emotional responsiveness task as quite boring and found it difficult to keep their attention with the task. However, our data do not allow determining whether lack of differential self-report ratings was due to experiencing difficulties in explicitly assigning these ratings or not complying with task instructions. Nevertheless, EMG data showed to be sensitive to picture valence, as indicated by differences in responses to positive and negative stimuli. A third limitation concerns the ceiling of 20 min in the SETT. A substantial number of participants did not reach pain tolerance within this time frame, making tolerance a less reliable outcome. Additionally, one might argue that it would have been valuable to study general affective switching performance in relation to pain and recovery from pain, rather than the different types of switch costs. Weak to moderate correlations between the types of switch costs were found in the current study, which replicates previous research and suggests that these switch costs tap into different components of affective flexibility (Genet et al. 2013; Malooly et al. 2013). Different emotion regulation strategies such as rumination (Genet et al. 2013) and reappraisal (Malooly et al. 2013) have been shown to be related to specific types of switch costs. Therefore, these findings stress that it is worthwhile to distinguish between the different types of switch costs. Lastly, we did not include a measure of general cognitive flexibility (Kashdan and Rottenberg 2010). Poorer cognitive flexibility has been shown to be predictive of chronic postsurgical pain (Attal et al. 2014) and may be another interesting factor to study in the context of pain experience. Future studies could therefore include measures of both EF and cognitive flexibility to investigate the unique and combined predictive value of these concepts for pain experience and recovery. Additionally, the fact that this study only found evidence for associations between two specific measures of EF and recovery from pain intensity and pain unpleasantness requires replication in future studies. Lastly, generalizability of this study is limited, as we subjected pain-free healthy students, of which the majority was female, to an experimental pain procedure.
In sum, this study is the first to indicate associations between two aspects of EF (i.e. flexible emotional responsiveness and affective flexibility) and recovery from pain in terms of pain intensity and pain unpleasantness. More flexibility in emotional responsiveness was found related to faster recovery from pain unpleasantness. In addition, difficulties in switching toward the positive aspects of positive stimuli were found associated with slower recovery regarding pain intensity. No associations were observed between any of the EF measures and pain tolerance. Therefore, we suggest that EF may particularly play a role in how people recover from pain. These data afford novel insights into EF as a potential factor elucidating how people manage their pain and recover from it. Only when protective factors are studied in addition to risk factors, we will be able to formulate scientific theories that allow a comprehensive understanding of pain perception and chronicity. Extending present theoretical models with EF may further our knowledge about why some people are more prone to develop chronic pain, which may ultimately lead to tailor-made prevention and treatment programs. For instance, EF training prior to surgery might be a useful method in the prevention of slow post-operative recovery. In order to explore this potential, future studies should examine whether it would be possible to train EF and its different aspects. In this respect, studies on the trainability of cognitive flexibility already showed promising results (e.g. Grönholm-Nyman et al. 2017; Zhao et al. 2018). Further, current treatments for chronic pain such as mindfulness-based interventions that aim at increasing EF and/or cognitive flexibility (Gallant 2016; Moynihan et al. 2013; Ortner et al. 2007) offer interesting new angles for future research on the relationship between EF and pain.
Notes
After outlier correction on DiffAffect, similar results were found for the regression analyses including and excluding DiffAffect as a predictor. In addition, DiffAffect never significantly contributed to the regression models.
The proportion of correct responses was higher for repeat trials (M = 9.15, SD = 6.12) than for switch trials (M = 6.16, SD = 4.77) t(51) = − 6.95, p < .001, d = 1.95, supporting the absence of a speed-accuracy trade-off.
References
Aldao, A., Sheppes, G., & Gross, J. J. (2015). Emotion regulation flexibility. Cognitive Therapy and Research, 39(3), 263–278. https://doi.org/10.1007/s10608-014-9662-4.
Aron, E. N., & Aron, A. (1997). Sensory-processing sensitivity and its relation to introversion and emotionality. Journal of Personality and Social Psychology, 73(2), 345–368.
Asmundson, G. J., Bovell, C. V., Carleton, R. N., & McWilliams, L. A. (2008). The Fear of Pain Questionnaire-Short Form (FPQ-SF): Factorial validity and psychometric properties. Pain, 134(1–2), 51–58. https://doi.org/10.1016/j.pain.2007.03.033.
Attal, N., Masselin-Dubois, A., Martinez, V., Jayr, C., Albi, A., Fermanian, J., … Baudic, S. (2014). Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain, 137(Pt 3), 904–917. https://doi.org/10.1093/brain/awt354.
Beshai, S., Prentice, J. L., & Huang, V. (2018). Building blocks of emotional flexibility: Trait mindfulness and self-compassion are associated with positive and negative mood shifts. Mindfulness, 9(3), 939–948. https://doi.org/10.1007/s12671-017-0833-8.
Bjekić, J., Živanović, M., Purić, D., Oosterman, J. M., & Filipović, S. R. (2018). Pain and executive functions: A unique relationship between Stroop task and experimentally induced pain. Psychological Research Psychologische Forschung, 82(3), 580–589. https://doi.org/10.1007/s00426-016-0838-2.
Block, J., & Kremen, A. M. (1996). IQ and ego-resiliency: Conceptual and empirical connections and separateness. Journal of Personality and Social Psychology, 70(2), 349–361.
Bonanno, G. A., Papa, A., Lalande, K., Westphal, M., & Coifman, K. (2004). The importance of being flexible: The ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychological Science, 15(7), 482–487. https://doi.org/10.1111/j.0956-7976.2004.00705.x.
Bond, F. W., Hayes, S. C., Baer, R. A., Carpenter, K. M., Guenole, N., Orcutt, H. K., … Zettle, R. D. (2011). Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: A revised measure of psychological inflexibility and experiential avoidance. Behavior Therapy, 42(4), 676–688. https://doi.org/10.1016/j.beth.2011.03.007.
Boselie, J. J., Vancleef, L. M., Smeets, T., & Peters, M. L. (2014). Increasing optimism abolishes pain-induced impairments in executive task performance. Pain, 155(2), 334–340. https://doi.org/10.1016/j.pain.2013.10.014.
Bylsma, L. M., Morris, B. H., & Rottenberg, J. (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. https://doi.org/10.1016/j.cpr.2007.10.001.
Cacioppo, J. T., Petty, R. E., Losch, M. E., & Kim, H. S. (1986). Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology, 50(2), 260–268.
Campbell-Sills, L., & Stein, M. B. (2007). Psychometric analysis and refinement of the Connor–Davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20(6), 1019–1028. https://doi.org/10.1002/jts.20271.
Chida, Y., & Hamer, M. (2008). Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin, 134(6), 829–885. https://doi.org/10.1037/a0013342.
Craig, A. D. (2003). A new view of pain as a homeostatic emotion. Trends in Neurosciences, 26(6), 303–307.
Eccleston, C., & Crombez, G. (1999). Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychological Bulletin, 125(3), 356–366.
Fledderus, M., Oude Voshaar, M. A., ten Klooster, P. M., & Bohlmeijer, E. T. (2012). Further evaluation of the psychometric properties of the Acceptance and Action Questionnaire-II. Psychological Assessment, 24(4), 925–936. https://doi.org/10.1037/a0028200.
Fridlund, A. J., & Cacioppo, J. T. (1986). Guidelines for human electromyographic research. Psychophysiology, 23(5), 567–589.
Gallant, S. N. (2016). Mindfulness meditation practice and executive functioning: Breaking down the benefit. Consciousness and Cognition, 40, 116–130. https://doi.org/10.1016/j.concog.2016.01.005.
Genet, J. J., Malooly, A. M., & Siemer, M. (2013). Flexibility is not always adaptive: Affective flexibility and inflexibility predict rumination use in everyday life. Cognition & Emotion, 27(4), 685–695. https://doi.org/10.1080/02699931.2012.733351.
Genet, J. J., & Siemer, M. (2011). Flexible control in processing affective and non-affective material predicts individual differences in trait resilience. Cognition & Emotion, 25(2), 380–388. https://doi.org/10.1080/02699931.2010.491647.
Grol, M., & De Raedt, R. (2017). The effect of positive mood on flexible processing of affective information. Emotion. https://doi.org/10.1037/emo0000355.
Grönholm-Nyman, P., Soveri, A., Rinne, J. O., Ek, E., Nyholm, A., Stigsdotter Neely, A., & Laine, M. (2017). Limited effects of set shifting training in healthy older adults. Frontiers in Aging Neuroscience. https://doi.org/10.3389/fnagi.2017.00069.
Gross, J. J. (2007). Handbook of emotion regulation. New York: Guilford Press.
Hollenstein, T., Lichtwarck-Aschoff, A., & Potworowski, G. (2013). A model of socioemotonal flexibility at three time scales. Emotion Review, 5, 397–405. https://doi.org/10.1177/1754073913484181er.sagepub.com.
Karp, J. F., Reynolds, C. F. 3rd, Butters, M. A., Dew, M. A., Mazumdar, S., Begley, A. E., … Weiner, D. K. (2006). The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Medicine, 7(5), 444–452. https://doi.org/10.1111/j.1526-4637.2006.00212.x.
Karsdorp, P. A., Geenen, R., & Vlaeyen, J. W. (2014). Response inhibition predicts painful task duration and performance in healthy individuals performing a cold pressor task in a motivational context. European Journal of Pain, 18(1), 92–100. https://doi.org/10.1002/j.1532-2149.2013.00348.x.
Kashdan, T. B., & Rottenberg, J. (2010). Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review, 30(7), 865–878.
Keefe, F. J., Lumley, M., Anderson, T., Lynch, T., Studts, J. L., & Carson, K. L. (2001). Pain and emotion: New research directions. Journal of Clinical Psychology, 57(4), 587–607.
Koechlin, H., Coakley, R., Schechter, N., Werner, C., & Kossowsky, J. (2018). The role of emotion regulation in chronic pain: A systematic literature review. Journal of Psychosomatic Research, 107, 38–45. https://doi.org/10.1016/j.jpsychores.2018.02.002.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1990). Emotion, attention, and the startle reflex. Psychological Review, 97(3), 377–395.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): Technical manual and affective ratings. Gainesville: NIMH Center for the Study of Emotion and Attention, University of Florida.
Larsen, J. T., Norris, C. J., & Cacioppo, J. T. (2003). Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology, 40(5), 776–785.
Malooly, A. M., Genet, J. J., & Siemer, M. (2013). Individual differences in reappraisal effectiveness: The role of affective flexibility. Emotion, 13(2), 302–313. https://doi.org/10.1037/a0029980.
Moore, P. A., Duncan, G. H., Scott, D. S., Gregg, J. M., & Ghia, J. N. (1979). The submaximal effort tourniquet test: Its use in evaluating experimental and chronic pain. Pain, 6(3), 375–382.
Moynihan, J. A., Chapman, B. P., Klorman, R., Krasner, M. S., Duberstein, P. R., Brown, K. W., & Talbot, N. L. (2013). Mindfulness-based stress reduction for older adults: Effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology, 68(1), 34–43. https://doi.org/10.1159/000350949.
Oosterman, J. M., Dijkerman, H. C., Kessels, R. P., & Scherder, E. J. (2010). A unique association between cognitive inhibition and pain sensitivity in healthy participants. European Journal of Pain, 14(10), 1046–1050. https://doi.org/10.1016/j.ejpain.2010.04.004.
Ortner, C. N. M., Kilner, S. J., & Zelazo, P. D. (2007). Mindfulness meditation and reduced emotional interference on a cognitive task. Motivation and Emotion, 31(4), 271–283. https://doi.org/10.1007/s11031-007-9076-7.
Papousek, I., Reiser, E. M., Weber, B., Freudenthaler, H. H., & Schulter, G. (2012). Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology, 49(4), 489–498. https://doi.org/10.1111/j.1469-8986.2011.01324.x.
Sarason, I. G., Johnson, J. H., & Siegel, J. M. (1978). Assessing the impact of life changes: Development of the life experiences survey. Journal of Consulting and Clinical Psychology, 46(5), 932–946.
Scheier, M. F., Carver, C. S., & Bridges, M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the life orientation test. Journal of Personality and Social Psychology, 67(6), 1063–1078. https://doi.org/10.1037//0022-3514.67.6.1063.
Smith, G. M., Egbert, L. D., Markowitz, R. A., Mosteller, F., & Beecher, H. K. (1966). An experimental pain method sensitive to morphine in man: The submaximum effort tourniquet technique. Journal of Pharmacology and Experimental Therapeutics, 154(2), 324–332.
Spielberger, C. D., Gorsuch, R. L., Lushenne, R. E., Vagg, P. R., & Jacobs, G. A. (1983). Manual for the Stait-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press.
Sullivan, M. J. L. (2008). Toward a biopsychomotor conceptualization of pain: Implications for research and intervention. Clinical Journal of Pain, 24, 281–290. https://doi.org/10.1097/AJP.0b013e318164bb15.
Sullivan, M., Bishop, S. R., & Pivik, J. (1995). The Pain Catastrophizing Scale: Development and validation. Psychological Assessment, 7(4), 524–532. https://doi.org/10.1037/1040-3590.7.4.524.
Tassinary, L. G., & Cacioppo, J. T. (1992). Unobservable facial actions and emotion. Psychological Science, 3(1), 28–33. https://doi.org/10.1111/j.1467-9280.1992.tb00252.x.
Tassinary, L. G., Cacioppo, J. T., & Vanman, E. J. (2007). The skeletomotor system: Surface electromyography. In J. T. Cacioppo, L. G. Tassinary & G. Berntson (Eds.), Handbook of psychophysiology (3 ed., pp. 267–300). Cambridge: Cambridge University Press.
Van Damme, S., Crombez, G., & Eccleston, C. (2004). The anticipation of pain modulates spatial attention: Evidence for pain-specificity in high-pain catastrophizers. Pain, 111(3), 392–399. https://doi.org/10.1016/j.pain.2004.07.022.
Van Ryckeghem, D. M., Crombez, G., Eccleston, C., Liefooghe, B., & Van Damme, S. (2012). The interruptive effect of pain in a multitask environment: An experimental investigation. The Journal of Pain, 13(2), 131–138. https://doi.org/10.1016/j.jpain.2011.09.003.
Verdejo-Garcia, A., Lopez-Torrecillas, F., Calandre, E. P., Delgado-Rodriguez, A., & Bechara, A. (2009). Executive function and decision-making in women with fibromyalgia. Archives of Clinical Neuropsychology, 24(1), 113–122. https://doi.org/10.1093/arclin/acp014.
Waugh, C. E., Thompson, R. J., & Gotlib, I. H. (2011). Flexible emotional responsiveness in trait resilience. Emotion, 11(5), 1059–1067. https://doi.org/10.1037/a0021786.
Westphal, M., Seivert, N. H., & Bonanno, G. A. (2010). Expressive flexibility. Emotion, 10(1), 92–100. https://doi.org/10.1037/a0018420.
Zhao, X., Wang, H., & Maes, J. H. R. (2018). Training and transfer effects of extensive task-switching training in students. Psychological Research. https://doi.org/10.1007/s00426-018-1059-7.
Acknowledgements
We thank Dr. Christian Waugh for kindly providing us the task set up and selected picture sets for the emotional responsiveness task. We also thank Dr. Ashley Malooly and Dr. Matthias Siemer for kindly providing us the materials for the affective switching task.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the standards of the ethical review committee of the Faculty of Psychology and Neuroscience (ERCPN), Maastricht University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Meesters, A., Vancleef, L.M.G. & Peters, M.L. Emotional flexibility and recovery from pain. Motiv Emot 43, 493–504 (2019). https://doi.org/10.1007/s11031-018-9748-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11031-018-9748-5