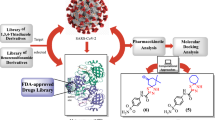
Abstract
New and facile one-pot approach for the syntheses of 12 derivatives of 3,5-disubstituted furane-2(5H)-one (4a–l) from easily available starting materials. The suitable synthetic procedures for selective synthesis of diverse furane-2(5H)-one derivatives were achieved via multi-component condensation of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1), pyruvic acid and different aromatic amines 3a–l in good to high yields and short reaction time by refluxing in acetic acid as well as obtained by another method (method B) when unsaturated arylidene pyruvic acid 6 was refluxed with different aromatic amines in acetic acid but in smaller yield than method A. Structures of the prepared compounds were elucidated by elemental analysis and spectral data as mass, IR, 1H-NMR and 13C-NMR spectroscopy. The antiviral efficacy of compounds 4a–l against SARS-CoV-2 was evaluated using the MTT assay. It was demonstrated that synthetic compounds 4c–e and 4h–j have a potent and selective inhibitory effect on SARS-CoV-2, a strain obtained from Egyptian patients. We utilized density-functional theory (DFT) analyses to deduce the molecular structures and topologies of the more energetic molecules. Molecular docking studies were performed against the SARS-CoV-2 main protease (PDB ID: 6Y84) and the SARS-CoV-2 Nsp9 RNA binding protein (PDB ID: 6W4B) to study the binding mechanism, non-bonding interactions, and binding affinity. Lastly, a hypothetical pharmacophore model was constructed by applying the Molecular Operating Environment (MOE) tool and eleven pharmaceuticals with proven antiviral activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Furan-2(5H)-ones have been identified as a valuable target for various organic chemists due to their presence as a subunit in several natural products isolated from a range of sources such as sponges, algae [1], animals [2], plants [3], and insects [4]. This core unit is essential for inducing a wide range of biological actions such as antibacterial [5, 6], antifungal [7, 8], anti-inflammatory [9], anticancer [10, 11], and antiviral HIV [11]. Butenolide synthon is a key structural unit present in numerous bioactive natural compounds, including Rubrolide 1 and Sarcophine 2, both derived from the colonial tunicate Ritterela rubra [12], as well as the synthetic medicine Benfurodil hemisuccinate 3 (Fig. 1).
On the other hand, substances that include a butenolide unit have a variety of biological effects, including phospholipase A2 inhibition and cyclo-oxygenase, as well as anticancer, antibiotic, anti-inflammatory, insecticidal, fungicidal, and bactericidal effects [13,14,15,16] (Fig. 2). The structural core shared by many naturally occurring molecules is the key framework of γ-butenolide and γ-butyrolactone [17,18,19]. The biological activity of γ-butenolides, γ-butyrolactones, and their derivatives are remarkable, particularly when they are available in enantiomerically pure form [20]. Additionally, they are crucial for the creation of physiological and medicinal agents. These are also very useful synthetic intermediates in the total synthesis of natural compounds. One of the products of the Doebner protocol is the synthesis of derivatives containing a butenolide nucleus. Doebner discovered the first multi-component pyruvic acid reaction in the late nineteenth century [21, 22]; it was a condensation of three-components of pyruvic acid, aldehydes, and derivatives of aniline which was produced either pyrrolidine-2,3-diones or quinoline carboxylic acids (Scheme 1).
Additionally, this reaction was thoroughly investigated in several articles [23,24,25]. Initially, it was thought that the Doebner reaction took place utilizing arylidenepyruvic acid production [26]. Still, this idea was subsequently debunked, and an alternative molecular route was proposed [27], which included the synthesis of a Schiff base and subsequent cyclization. Despite previous reports to the contrary, 3-arylamino-5-aryl-2(5H) furanones [28] were the product of the interaction between arylidenepyruvic acids and aromatic amines, rather than pyrrolidine-2,3-diones [29] (Scheme 1). It is important to note that various Doebner reaction protocols have been created [24, 25] depending on the change of solvents, catalysts, and activation techniques.
Keeping with our prior work on biomimetic methods [30,31,32,33], looking at the butanolide synthon’s biological activity and the limitations of earlier reactions [34, 35], we report herein the synthesis of 3,5-disubstituted furane-2(5H)-one derivatives (4a–l) through a facile and simple method, via multi-component condensation of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) [36], pyruvic acid and different aromatic amines 3a–l. The derivative of pyrazole-4-carbaldehyde was generated as previously reported [37] via the Vilsmeier–Haack reaction on the associated hydrazone derivative. Compounds 4c–e, and 4h–j showed high to moderate activity when evaluated in vitro against the SARS-CoV-2 strain recovered from Egyptian patients.
The use of density functional theory (DFT) methods, particularly hybrid functional approaches, has grown into a reliable and efficient tool for the determination of many molecular characteristics. An investigation of the examined molecules’ HOMO and LUMO orbitals was carried out using frontier molecular orbitals (FMOs), which helped to shed light on details about charge transfer [38]. Using molecular electrostatic potential (MEP) analysis, the electron distribution and the identification of active nucleophilic and electrophilic sites of the compounds in concern were determined. Molecular docking experiments were also conducted against the SARS-CoV-2 main protease (PDB ID: 6Y84) and the SARS-CoV-2 Nsp9 RNA binding protein (PDB ID: 6W4B) to explain the antiviral action of the target compounds by showing the likely binding manner of the molecules with their target proteins. Lastly, the paper focused on the antiviral activity of the more active compounds and how their pharmacophoric model was calculated.
Results and discussion
Chemistry
Prior research examined many pyruvic acid and polyfunctional aminoazole reactions that were sequential and linear in nature, focusing on these reactions from the perspectives of the selectivity and chemical diversity of the reactants [39,40,41]. The presentation focused on how structural parameters, activation technique, temperature, and catalytic system affected the trend of the reactions. The selectivity of pyruvic acid [40, 41] and other heterocyclization [42, 43] processes may be controlled by varying these parameters. Aniline, an aromatic amine, typically forms a pyridine ring with the presence of an NH2 group in multi-component reactions involving aldehydes and active methylene compounds (CH acids), as previously described by the Doebner Reaction in the late nineteenth century [21, 22]. Also, several articles [23] examined this response in depth.
This article details a novel approach to multi-component management of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde [36] with suitable aromatic amines and pyruvic acid or its linear interactions with arylidene pyruvic acids to produce several forms of pyrazolo furan-2(5H)-one. It was established that three-component interaction of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) [36] with pyruvic acid (2) and appropriate aromatic amine in boiling acetic acid occurred in an unconventional manner and produced an unprecedented pyrazolo furan-2(5H)-one (4) as the only product in yields ranging from 68% to 83% (Method A, Scheme 1).
The synthesis of pyrazolo furan-2(5H)-one derivatives (4a–l) involves a multi-component process that follows two routes, as previously described [44]. One of the pathways involves treating pyruvic acid with 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) [36] for the first time, which results in the synthesis of arylidene pyruvic acid 6. An interaction between aromatic amine 3a–l and arylidenepyruvic acid 6 can lead to the creation of imine 7 and its subsequent cyclization into final heterocycle 4a–l (route A). A different possible route involves the following steps: the aromatic amine’s NH2 group attacks the β-carbonyl group of pyruvic acid, forming imine 8. Then, the corresponding furanone derivatives 4a–l are produced through cyclization with 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) [36] (Route B) (Scheme 2). Route A was used to chemically confirm the production of furanone derivatives (Method B, Scheme 1).
The flow of treatment was the same for a sequential reaction, including the first synthesis of arylidene pyruvic acids, as it was for the multi-component process. Consequently, facile and simple methods for the formation of pyrazolo furan-2(5H)-one (4a–l) via multi-component reaction of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) [36], pyruvic acid (2) and aromatic amine (3a–l) in acetic acid was synthesized (Method A, Scheme 3) and confirmed chemically by methods B (T.L.C. with authentic sample), as well as by spectroscopic date.
The structure of the resulting pyrazolo furan-2(5H)-one derivatives (4a–l) were checked considering their IR, Mass, 1H NMR, 13C NMR spectroscopy, and elemental analyses. For example, the infra-red spectra of compounds (4a–l) clearly showed the characteristic NH groups at the range of (3370–3395) cm−1, as well as showed the characteristic peaks of carbonyl groups at the range of (1684–1751) cm−1. Their 1H NMR spectra were in complete agreement with the assigned chemical structures which showed doublet of 5-CH furanone at the range of δ = (5.13–7.33) ppm, J = (6.1–8.9 Hz), doublet of 4-CH furanone at the range of δ = (6.88–7.59) ppm, J = (7.4–9.4 Hz), singlet of CH pyrazole at the range of δ = (7.55–8.56) ppm, and singlet of NH proton at the range of δ = (9.12–9.40) ppm (exchangeable by D2O), besides appropriate signals of terminal substituents. The 13C NMR spectra of compounds (4a–l) showed a signal at the range of δ = (163.71–173.64) ppm corresponding to the (C=O) group in addition to the appropriate chemical shift of aromatic carbons.
Antiviral activity
The half-maximal inhibitory concentration (IC50)
A potential antiviral that targets SARS-CoV-2 might be developed and administered to patients at an early stage of illness. This would assist in reducing the viral load, halting the course of severe disease, and restricting the spread of the virus from person to person. Consequently, it is crucial to do Benchmark testing on these compounds as soon as possible in comparison to other possible SARS-CoV-2 antivirals with different action mechanisms [45].
Different concentrations of 2, 4, 8.7, 17.5, 35, and 70 µM of the synthetic compounds sample 4a–l were evaluated in vitro against the SARS-CoV-2 strain that was recovered from Egyptian patients. Table 1 and Fig. 3 describe the results, which are shown as IC50 values. Using MTT assay [46], we examined the impact of various compound doses on the proliferation of the Vero E6 cell line after 24 h of treatment. Most of the derivatives examined exhibited cytotoxic action against SARS-CoV-2, ranging from moderate to excellent, with concentrations ranging from 1.8 to 150 µM, as indicated by the findings.
Plaque reduction assay (% of inhibition SARS-CoV-2)
Table 2 and Fig. 4 reveal that samples 4c–e and 4h–j exhibited strong activity against the SARS-CoV-2 that was isolated from patients in Egypt. Out of all the derivatives tested, the one with the highest percentage of inhibition (78% and 77%) against viral reproduction was 4c, which had 2-chloroaniline as its aromatic amine, and 4e, which contained aniline. Also, the activity dropped to 68%, 66%, and 63% when the aromatic amine was substituted with 2-aminophenol, 4-chloroaniline, 4-bromoaniline, or p-anisidine, as shown in examples 4d and 4h–j, respectively. A weak antiviral activity against SARS-CoV-2 was demonstrated by samples 4a,b, 4f,g, and 4k,l, where the aromatic amine was 3-methyl 2-aminobenzoate, o-anisidine, m-anisidine, p-toluidine, α-naphthyl amine, or ethyl 5-amino-1-phenyl-1H-pyrazole-4-carboxylate in which the percentage of inhibition ranged from 33% to 58%.
Computational study of the DFT calculations
To study the electronic structure of the most powerful molecules 4c–e and 4h–j, computational quantum mechanical modeling is employed using density functional theory (DFT). Utilizing the DFT basis set, the electrical and structural properties of the optimized compounds 4c–e and 4h–j (Fig. 5) were measured. This study makes use of the following computed parameters: chemical hardness, chemical softness, electronegativity, electrophilic index, energies of the highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbitals, and the HOMO–LUMO energy gap. Gauss View was used to view the molecular electrostatic potential surfaces (MEPs) that were derived from the population analysis calculations. The intensity of the interaction between ligands and the binding pocket of the COVID-19 main protease is explained in large part by these factors.
Molecular electrostatic potential (MEP)
With MEP, researchers can see the size and geometry of molecules as well as the neutral positive, and negative electrostatic potential zones all at once, according to its colored grading system. The title compounds 4c–e and 4h–j MEP maps were built using the optimized findings of the Gaussian-09W set. MEP was calculated to foretell the electrophilic and nucleophilic attack sites in the optimal configuration of compounds that were tested (Fig. 6). Based on the arrangement of colors; the potential grows according to the following sequence: red, orange, yellow, green, and finally, blue. The red area represents the largest negative area, which is a promising target for electrophilic attacks. The blue area is the most positively charged, suggesting a prime spot for nucleophilic attacks. Green suggests a potential middle ground between the two extremes (red and dark blue). Intervening between the green for moderate and the red/dark blue for extreme, you have the yellow and light blue colors.
In Fig. 6, the carbonyl group’s oxygen and the furanone ring’s oxygen atom are examples of electron-rich regions. In contrast, the nitrogen of the NH bridge connected to furanone derivatives (4c–e, and 4h–j), the hydroxyl at 4d, CH-of all pyrazole, furanone, and phenyl ring for the target derivatives are examples of electron-deficient regions. Additionally, the nitrogen atoms of the pyrazole ring, Cl at 4c,h, Br at 4i, and OCH3 at 4j, which represent neutral sites, take on yellow and green colors. Based on a molecular docking study, the MEP of target derivatives 4c–e and 4h–j is rich with positive and negative regions that play a significant role in the interaction with biological targets. The hydrogen bonds formed between these regions, whether as donors or acceptors, differ between the active site and the pocket, as shown in Table 5.
Frontier molecular orbitals
Quantum chemistry calculations using the frontier molecular orbitals (FMOs) are essential for predicting the stability, and reactivity [47, 48] of the compounds. In molecular orbitals, the LUMO represents an empty state that can take electrons, while the HOMO represents a state where an electron may be donated (complete stage). The transition from the ground state to the first excited state [49] is determined by the excitation of one electron from the HOMO to the LUMO. The wider the HOMO–LUMO gap, the more stable the kinetics are. The FMOs are shown graphically in Fig. 7. The energy values are EHOMO = (−0.21789, −0.20013, −0.20631, −0.21452, −0.21220 and −0.19482) eV, ELUMO = (−0.04516, −0.03921, −0.04904, −0.05571, −0.05488 and −0.04646) eV and energy gap is ∆E = (0.17273, 0.16092, 0.15727, 0.15881, 0.15732 and 0.14836) eV for compounds 4c–e, and 4h–j, respectively.
The use of HOMO and LUMO is employed to ascertain molecular charge distributions, intramolecular charge transfers, and electronic transitions of molecules. The electronegativity (χ), electrophilic index (ω), chemical hardness (ɳ), and chemical softness (S) of compounds 4c–e, and 4h–j may be determined by utilizing the HOMO and LUMO orbital energies according to the following equations:
With respect to Table 3, Compounds 4c–e, and 4h–j have electronegativity values of χ = (0.13152, 0.11967, 0.12767, 0.13511, 0.13354 and 0.12064) eV, respectively, which illustrate the tendency of electrons to depart from a stable system. ɳ = (0.08636, 0.08046, 0.07863, 0.07940, 0.07866 and 0.07418) eV, are the chemical hardness values for compounds 4c–e, and 4h–j, respectively, this parameter quantifies the resistance to changes in the electron distribution and is linked to the reactivity of the chemical system. The electrophilicity index values are as follows: ω = (0.10014, 0.08899, 0.10364, 0.11495, 0.11335 and 0.09809) eV, respectively; these values indicate the degree of energy reduction caused by the maximum electron flow occurring between the acceptor and donor. The energy gap provides insight into the chemical stability and reactivity of the compounds, as estimated from the reactivity descriptors in the table. The compounds’ limited stability and high reactivity are demonstrated by the narrow energy gap between their orbitals. Compounds 4c–e, and 4h–j are also chemically reactive, as seen by their reduced chemical hardness and increased chemical softness.
Visualization of the binding mode
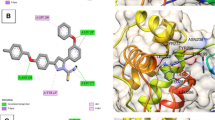
Exploring protein–ligand interactions by molecular docking with several computer-assisted drug design tools can lead to the development of a therapeutic agent with great promise for the treatment of certain diseases. So, we’re using molecular docking to find out how well pyrazolyl furanone derivatives 4c–e, and 4h–j block SARS-CoV-2 main protease (PDB ID: 6Y84) and Nsp9 RNA binding protein (PDB ID: 6W4B). Table 5 shows the interactions between amino acids, the lengths of hydrogen bonds in Å, and the affinity in kcal/mol for the bonds.
The docking investigations showed that compounds 4c–e, and 4h–j interacted strongly with (6Y84) and (6W4B) of SARS-CoV-2. The predicted binding energies for (6Y84) were from −6.56 to −7.98 kcal/mol, and for (6W4B), they were in the range of −5.53 to −6.59 kcal/mol, indicating that the prepared compounds are capable of spontaneously interacting in the vicinity of the receptors selected by SARS-CoV-2.
Docking on the receptor SARS-CoV-2 main protease (PDB ID: 6Y84)
Significant interactions and binding of the compounds with the protein (6Y84) were observed subsequent to the successful docking of the promising compounds 4c–e, and 4h–j with the SARS-CoV-2 main protease (PDB ID: 6Y84) (Tables 4 and 5). −7.21 kcal/mol, −6.89 kcal/mol, −6.56 kcal/mol, −7.68 kcal/mol, −6.81 kcal/mol, and −7.98 kcal/mol, respectively, are the binding energies (∆G) of the more powerful compounds 4c–e, and 4h–j.
In all the tested compounds, the residue Arg 298 forms an electrostatic pi-cation and arene-H contact with the phenyl ring. Also, compound 4c revealed one hydrogen bond with Gln 299 and an arene-H contact with Thr 304 (Fig. 8). The proposed binding pattern of compound 4d revealed two H-bonds with Tyr 154 and an arene-H contact with Thr 304. Compound 4e was combined with the receptor through one hydrogen bond with Gly 302, an H-arene contact with Phe 8 and two arene-H interaction with Thr 304 and Pro 9 (Fig. 9). Compound 4h stabilizes the connection with SARS-CoV-2 main protease (PDB ID: 6Y84) within a H-bond with Tyr 154 amino acid, H-arene contact with Phe 8, and two arene-H interaction with Thr 304 and Ile 152. Compound 4i forms two H-bonds with the residues Val 303 and Arg 298. In addition, it showed an arene-H interaction with Pro 9 and Thr 304 amino acids. Finally, compound 4j established three hydrogen bonds with Tyr 154, Met 6, and Val 303. In addition, the compound showed three arene-H interactions with Pro 9, and Ile 152 amino acids.
Docking on the receptor SARS-CoV-2 Nsp9 RNA binding protein (PDB ID: 6W4B)
According to docking tests conducted on the SARS-CoV-2 Nsp9 RNA binding protein (PDB ID: 6W4B), the active pocket orientations of the synthesized compounds are comparable (Tables 4 and 5). For the more powerful compounds 4c–e, and 4h–j, the binding energies (∆G) are −5.86 kcal/mol, −5.88 kcal/mol, −5.53 kcal/mol, −6.24 kcal/mol, −6.21 kcal/mol, and −6.59 kcal/mol, respectively.
The interaction of compound 4c against the target protein was confirmed with Arg 100 and Cys 74 through four hydrogen bonds, with Ace 5 through an arene-H interaction, and with Ser 6 amino acid through an electrostatic pi-cation contact (Fig. 10). Compound 4d was coupled with the receptor protein by forming two hydrogen bonds with Cys 74, an H-arene contact with Phe 76, and two arene-H interactions with the amino acid as Cys 74 and Pro 7. Compound 4e stabilized the connection with the protein by forming two H-bonds with Arg 75 and Cys 74 amino acids. In addition, it produces two arene-H contacts with Phe 91 and Pro 7 (Fig. 11). The expected binding pattern of compound 4h revealed the presence of two hydrogen bonds with residues of amino acids, including Cys 74. Further, an H-arene contact with Phe 76 and three arene-H contacts with the residues Cys 74, Ace 5, and Pro 7 were shown by 4h. Compound 4i was detected to form an H-bond with Ser 6 and two arene-H contacts with the residues Cys 74 and Arg 75. Finally, compound 4j revealed one hydrogen bond with Arg 75 and two arene-H interactions with Arg 75 and Cys 74 amino acids.
To ascertain the inhibitory potential of the substances under investigation 4c–e, and 4h–j against the main protease (PDB ID: 6Y84) and Nsp9 RNA binding protein (PDB ID: 6W4B) of SARS-CoV-2, their binding energy (∆G) values were contrasted with the binding energy (∆G) values of those of established antiviral medications [50], including Oseltamivir, Ritonavir, Remdesivir, Ribavirin, and Lopinavir, as well as Chloroquine, Mycophenolic acid (MPA), Isoniazid, Hydroxychloroquine (HCQ), Pemirolast, and Eriodictyol (Table 4).
Therefore, it is evident from Table 4 that in the case of docking against SARS-CoV-2 Nsp9 RNA binding protein (PDB ID: 6W4B), the binding energy (∆G) for the examined compounds was higher than ∆G of the most reported drugs and was close to the binding energy of Pemirolast, Eriodictyol, Lopinavir, and Remdesivir. While with the docking process against SARS-CoV-2 main protease (PDB ID: 6Y84), ∆G of the target compounds was higher than the ∆G of Isoniazid and Oseltamivir drugs but was close to the binding energy of Chloroquine and Hydroxychloroquine. Following this line of reasoning, it’s reasonable to assume that compounds 4c–e, and 4h–j possess inhibitory properties against SARC-CoV-2.
Pharmacophore studies
The objective of this methodology is to develop and assess a pharmacophore model (hypothesis) using SARS-CoV-2 inhibitors as a basis for the COVID-19 drug [50]. In 3D pharmacophore-based applications, three steps are commonly utilized: To start, the 11 SARS-CoV-2 inhibitors (Table 6) that will serve as training set compounds need to have their 3D structures built. Next, the pharmacophoric characteristics are given. In the end, a method is employed to search databases for new compounds 4c–e, and 4h–j that possess the specified pharmacophoric characteristics [51]. An indicator of a molecule’s activity is the degree to which it maps to a built hypothetical model; this is expressed as the rmsd (the root of the mean square distance), between the query features and their corresponding ligand-target sites (Table 7). Some of the most common pharmacophoric features are aromatic rings (Aro), hydrophobic groups (Hyd), charged or ionizable groups (Cat and Ani), metal ligators (ML), hydrogen bond acceptors, and donors (Acc and Don). Three features of the preliminary pharmacophoric assessment were determined, as graphically depicted in Fig. 12 and presented in Table 7.
According to Table 7, the rmsd’s inhibitory effect becomes stronger with decreasing values. Compound 4d superpositions with rmsd values of 0.2706 exhibited the most activity (Fig. 14). Aside from that, compounds 4c,e, and 4h–j exhibited strong inhibitory activity with corresponding rmsd values of 0.5165, 0.5295, 0.5207, 0.7083, and 0.4167 (Figs. 13, 15, 16, 17, and 18). The fact that the antiviral activity has shown encouraging effects is a hopeful sign.
Materials and methods
Chemistry
All compounds that were produced had their uncorrected melting points measured in open glass capillaries using a digital melting point apparatus from Electrothermal, the LA 9000 SERIS. The deuterated dimethyl sulfoxide (DMSO-d6) solvent was utilized to analyze the 1H,13C NMR spectra at (400 and 101) MHz with Varian Gemini and Bruker high performance digital FTNMR Avance III spectrometers. Mass spectra were acquired at 70 eV with a Schimadzu GC/MS-QP-5050A mass spectrometer at the Regional Center for Mycology and Biotechnology of Al-Azhar University. The Micro Analytical Unit of Cairo University conducted elemental analyses. The methyl 2-((1,3-diphenyl-1H-pyrazol-4-yl)-2-oxo-2,5-dihydrofuran-3-yl)amino)benzoate (4a) was synthesized initially using arylidene pyruvic acids 6 (m.p.: 182 °C; reported 184 °C [52]), and then cyclized with an aromatic amine 3a (Scheme 1, Method B). The compound 4a, which was produced in a yield of 68% using technique B and synthesized in two stages, was recognized by its spectroscopic analysis and melting point, both of which were identical to those obtained by method A.
General procedure for the synthesis of pyrazolo furan-2(5H)-one derivatives (4a–l)
Method (A)
A mixture of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) [36] (10 mmol, 2.48 g), pyruvic acid (10 mmol, ml 0.88), and the appropriate aromatic amine 3a–l (10 mmol) in 10 ml of acetic acid was refluxed for 10–40 min until a solid started to precipitate. After cooling, the crystals formed were collected by filtration, dried, and crystallized from EtOH.
Methyl 2-((5-(1,3-diphenyl-1H-pyrazol-4-yl)-2-oxo-2,5-dihydrofuran-3-yl)amino)benzoate (4a)
From 3-methyl 2-aminobenzoate, Yield: (A: 76, B: 68)% as yellow powder; m.p.: 118–120 °C; IR: ν/cm−1: 3465 (NH), 3061 (CH-Ar), 2995, 2950 (CH-aliph), 1710, 1693 (C=O), 1596 (C=N), 1449, 1063 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 3.60 (s, 3H, CH3), 6.76 (d, J = 8.4 Hz, 1H, 5-CH furanone), 7.40–7.45 (m, 2H, Ar H), 7.59 (d, J = 7.5 Hz, 3H, 2Ar H and 4-CH furanone), 7.71–7.75 (m, 6H, Ar H), 7.91 (dd, J = 7.1, 1.3 Hz, 2H, Ar H), 8.02 (d, J = 7.6 Hz, 2H), 8.21 (s, 1H, CH pyrazole), 9.22 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 52.48 (CH3), 81.13 (5-CH furanone), 112.29, 117.00, 118.67, 118.85, 119.21, 119.34, 119.48, 122.02, 122.76, 123.31, 129.17, 130.12, 131.04, 131.12, 131.59, 133.28, 133.37, 134.51, 135.37, 137.64, 139.61, 145.31, 151.47, 152.83, 167.72 (C=O); MS (m/z, %): 40.18 (59.32%), 80.17 (64.38%), 199.90 (92.28%), 358.48 (54.26%), 392.64 (100.00%), 451.62 (M+, 15.21%); Anal. Calcd. for C27H21N3O4 (451.48): C, 71.83; H, 4.69; N, 9.31; O, 14.17; Found: C, 71.80; H, 4.65; N, 9.26; O, 14.11.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-((2-methoxyphenyl)amino)furan-2(5H)-one (4b)
From o-anisidine, Yield: 73% as yellow powder; m.p.: 135–137 °C; IR: ν/cm−1: 3390 (NH), 3057 (CH-Ar), 2934, 2835 (CH-aliph), 1712 (C=O), 1598 (C=N), 1460, 1061 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 3.96 (s, 3H, OCH3), 6.74 (d, J = 7.8 Hz, 1H, 5-CH furanone), 7.24 (d, J = 7.8 Hz, 1H, 4-CH furanone), 7.40 (dd, J = 5.1, 2.0 Hz, 2H), 7.46–7.58 (m, 8H, Ar H), 7.90 (dd, J = 7.3, 2.3 Hz, 2H), 7.99 (s, 1H, CH pyrazole), 8.03 (d, J = 7.7 Hz,1H), 8.09 (d, J = 8.5 Hz, 1H), 9.19 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 57.38 (CH3), 79.68 (5-CH furanone), 119.25 (2C), 122.15, 123.58, 124.42, 126.31, 127.74, 127.92, 128.07, 128.45, 128.56, 128.70, 128.88, 129.19, 129.60, 129.72, 129.85 (2C), 131.25 (2C), 134.83, 138.59, 152.69, 172.29 (C=O); MS (m/z, %): 77.39 (100.00%), 91.72 (58.87%), 229.28 (40.40%), 296.50 (83.12%), 423.86 (M+, 17.25%); Anal. Calcd. for C26H21N3O3 (423.47): C, 73.74; H, 5.00; N, 9.92; O, 11.33; Found: C, 73.69; H, 4.93; N, 9.85; O, 11.27.
3-((2-Chlorophenyl)amino)-5-(1,3-diphenyl-1H-pyrazol-4-yl)furan-2(5H)-one (4c)
From 2-chloroaniline, Yield: 82% as yellow powder; m.p.: 115–117 °C; IR: ν/cm−1: 3372 (NH), 3059 (CH-Ar), 2924 (CH-aliph), 1712 (C=O), 1597 (C=N), 1447, 1060 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 6.71 (d, J = 8.3 Hz, 1H, 5-CH furanone), 7.09 (d, J = 8.0 Hz, 1H, 4-CH furanone), 7.37 (d, J = 6.3 Hz, 1H), 7.40 (dd, J = 5.0, 1.9 Hz, 2H), 7.47–7.86 (m, 8H, Ar H), 7.91 (d, J = 7.5 Hz, 1H), 8.03 (d, J = 7.8 Hz, 1H), 8.09 (s, 1H, CH pyrazole), 8.54 (d, J = 7.5 Hz, 1H), 9.28 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 76.00 (5-CH furanone), 118.61, 119.12 (2C), 122.29, 124.93, 127.24, 127.27, 127.87, 127.91, 128.45 (2C), 129.05, 129.65 (2C), 130.08, 130.82, 133.02, 133.33, 139.60, 144.07, 148.03, 151.76, 152.47, 167.74 (C=O); MS (m/z, %): 107.60 (100.00%), 105.39 (85.74%), 256.75 (87.72%), 282.61 (86.88%), 302.02 (73.59%), 427.31 (M+, 24.75%); Anal. Calcd. for C25H18ClN3O2 (427.89): C, 70.18; H, 4.24; Cl, 8.28; N, 9.82; O, 7.48; Found: C, 70.12; H, 4.17; Cl, 8.23; N, 9.75; O, 7.41.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-((2-hydroxyphenyl)amino)furan-2(5H)-one (4d)
From 2-aminophenol, Yield: 77% as brownish powder; m.p.: 183–185 °C; IR: ν/cm−1: 3395 (OH), 3224 (NH), 3062 (CH-Ar), 2970, 2926 (CH-aliph), 1733 (C=O), 1598 (C=N), 1453, 1061 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 7.33 (d, J = 7.6 Hz, 1H, 5-CH furanone), 7.38 (d, J = 8.6 Hz, 1H, 4-CH furanone), 7.42–7.49 (m, 4H, Ar H), 7.51 (d, J = 7.5 Hz, 2H), 7.53–7.58 (m, 4H, Ar H), 7.60 (s, 1H, CH pyrazole), 7.94 (d, J = 6.6 Hz, 2H), 8.00 (d, J = 7.9 Hz, 2H), 9.32 (s, 1H, NH exchangeable by D2O), 10.00 (s, 1H, OH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 77.70 (5-CH furanone), 119.25 (2C), 122.15, 127.74, 127.92, 128.18, 128.45, 128.56 (2C), 128.70 (2C), 128.88, 128.99, 129.19, 129.46, 129.60, 129.72, 129.85 (2C), 131.25, 134.83, 138.59, 152.59, 172.31(C=O); MS (m/z, %): 46.03 (100.00%), 52.39 (84.70%), 120.90 (80.14%), 159.58 (88.55%), 259.68 (83.54%), 409.52 (M+, 17.48%); Anal. Calcd. for C25H19N3O3 (409.45): C, 73.34; H, 4.68; N, 10.26; O, 11.72; Found: C, 73.30; H, 4.62; N, 10.21; O, 11.66.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-(phenylamino)furan-2(5H)-one (4e)
From aniline, Yield: 79% as yellow powder; m.p.: 114–116 °C; IR: ν/cm−1: 3386 (NH), 3059 (CH-Ar), 2926 (CH-aliph), 1712 (C=O), 1597 (C=N), 1449, 1061 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 6.68 (d, J = 8.3 Hz, 1H, 5-CH furanone), 7.30 (d, J = 7.9 Hz, 1H, 4-CH furanone), 7.40–7.58 (m, 9H, Ar H), 7.71 (dd, J = 6.4, 2.7 Hz, 2H, Ar H), 7.81 (d, J = 6.9 Hz, 1H, Ar H), 7.88 (d, J = 8.3 Hz, 1H, Ar H), 7.93 (d, J = 8.4 Hz, 1H, Ar H), 8.05 (s, 1H, CH pyrazole), 8.64 (d, J = 8.2 Hz, 1H, Ar H), 9.27 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 78.12 (5-CH furanone), 112.84, 119.08 (2C), 122.25, 127.84, 127.89, 128.54, 128.78 (2C), 129.03, 129.16, 129.22, 129.41, 129.64, 130.09, 130.20 (2C), 130.51, 133.42, 139.66, 148.75, 151.39, 152.20, 167.91 (C=O); MS (m/z, %): 82.97 (65.80%), 194.44 (66.36%), 233.68 (57.42%), 351.09 (100.00%), 392.95 (M+-1, 30.38%); Anal. Calcd. for C25H19N3O2 (393.45): C, 76.32; H, 4.87; N, 10.68; O, 8.13; Found: C, 76.25; H, 4.873; N, 10.62; O, 8.07.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-((3-methoxyphenyl)amino)furan-2(5H)-one (4f)
From m-anisidine, Yield: 83% as yellow powder; m.p.: 130–132 °C; IR: ν/cm−1: 3377 (NH), 3061 (CH-Ar), 2931, 2835 (CH-aliph), 1710 (C=O), 1597 (C=N), 1452, 1038 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 3.90 (s, 3H, OCH3), 6.03 (d, J = 7.3 Hz, 1H, 5-CH furanone), 6.92 (d, J = 9.4 Hz, 1H, 4-CH furanone), 7.22–7.25 (m, 1H, Ar H), 7.26–7.28 (m, 3H, Ar H), 7.40 (d, J = 2.1 Hz, 1H), 7.42 (d, J = 1.7 Hz, 1H), 7.53 (s, 1H, Ar H), 7.55 (s, 1H, CH pyrazole), 7.67 (m, 3H, Ar H), 8.02 (d, J = 7.7 Hz, 2H), 8.56 (d, J = 9.3 Hz, 2H), 9.12 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 55.84 (OCH3), 73.96 (5-CH furanone), 118.99 (2C), 122.82, 124.99, 126.72, 127.11, 127.73 (2C), 128.59 (2C), 129.08, 129.16 (2C), 130.06, 133.45, 138.68, 139.69, 139.88, 140.91, 150.34, 151.05, 152.47, 160.41, 172.94 (C=O); MS (m/z, %): 43.78 (100.00%), 173.68 (79.33%), 201.21 (73.14%), 234.17 (68.54%), 262.18 (93.03%), 423.51 (M+, 20.18%); Anal. Calcd. for C26H21N3O3 (423.47): C, 73.74; H, 5.00; N, 9.92; O, 11.33; Found: C, 73.70; H, 4.93; N, 9.84; O, 11.25.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-(p-tolylamino)furan-2(5H)-one (4g)
From p-toluidine, Yield: 69% as yellow powder; m.p.: 110–112 °C; IR: ν/cm−1: 3371 (NH), 3057 (CH-Ar), 2931 (CH-aliph), 1707 (C=O), 1597 (C=N), 1449, 1060 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 2.14 (s, 3H, CH3), 6.56 (d, J = 8.4 Hz, 1H, 5-CH furanone), 6.88 (d, J = 8.3 Hz, 1H, 4-CH furanone), 7.36–7.44 (m, 3H, Ar H), 7.53–7.55 (m, 3H, Ar H), 7.68 (dd, J = 6.6, 3.0 Hz, 2H), 7.80 (d, J = 8.6 Hz, 2H), 7.87 (d, J = 1.1 Hz, 2H), 7.95 (s, 1H, CH pyrazole), 8.02 (d, J = 7.7 Hz, 2H), 9.20 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 21.82 (OCH3), 78.84 (5-CH furanone), 111.65, 112.96, 116.78, 118.49, 119.01, 120.73, 122.32, 126.26, 127.80, 128.49, 129.05, 129.32, 129.37, 129.76, 130.05, 133.43, 139.53, 139.65, 143.15, 147.37, 149.84, 151.22, 154.35, 168.02 (C=O); MS (m/z, %): 65.19 (56.32%), 77.35 (100.00%), 88.37 (48.59%), 92.11 (70.45%), 407.50 (M+, 7.86%); Anal. Calcd. for C26H21N3O2 (407.47): C, 76.64; H, 5.19; N, 10.31; O, 7.85; Found: C, 76.61; H, 5.14; N, 10.26; O, 7.79.
3-((4-Chlorophenyl)amino)-5-(1,3-diphenyl-1H-pyrazol-4-yl)furan-2(5H)-one (4h)
From 4-chloroaniline, Yield: 72% as yellow powder; m.p.: 119–121 °C; IR: ν/cm−1: 3421 (NH), 3062 (CH-Ar), 2924, 2854 (CH-aliph), 1684 (C=O), 1598 (C=N), 1494, 1062 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 6.66 (d, J = 8.9 Hz, 1H, 5-CH furanone), 7.09 (d, J = 8.8 Hz, 1H, 4-CH furanone), 7.27–7.31 (m, 3H, Ar H), 7.38–7.45 (m, 3H, Ar H), 7.50 (dd, J = 18.7, 6.7 Hz, 2H), 7.66 (dd, J = 6.5, 3.1 Hz, 2H), 7.86 (d, J = 5.8 Hz, 2H), 8.03 (dd, J = 17.9, 8.0 Hz, 2H), 8.56 (s, 1H, CH pyrazole), 9.25 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 81.90 (5-CH furanone), 114.91, 116.64, 119.71 (2C), 120.78 (2C), 123.17, 126.77, 126.95, 127.85 (2C), 128.30 (2C), 128.61 (2C), 129.06, 129.24, 130.00, 132.61, 135.22, 135.37, 140.97, 153.49, 173.64 (C=O); MS (m/z, %): 227.00 (99.88%), 353.43 (56.67%), 407.37 (95.76%), 416.28 (100.00%), 422.01 (52.99%), 427.45 (M+, 13.75%); Anal. Calcd. for C25H18ClN3O2 (427.89): C, 70.18; H, 4.24; Cl, 8.28; N, 9.82; O, 7.48; Found: C, 70.12; H, 4.17; Cl, 8.21; N, 9.77; O, 7.42.
3-((4-Bromophenyl)amino)-5-(1,3-diphenyl-1H-pyrazol-4-yl)furan-2(5H)-one (4i)
From 4-bromoaniline, Yield: 80% as yellow powder; m.p.: 123–125 °C; IR: ν/cm−1: 3370 (NH), 3059 (CH-Ar), 2970, 2924 (CH-aliph), 1709 (C=O), 1597 (C=N), 1449, 1069 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 6.62 (d, J = 8.7 Hz, 1H, 5-CH furanone), 7.21 (d, J = 8.7 Hz, 1H, 4-CH furanone), 7.42 (dd, J = 11.1, 3.1 Hz, 2H), 7.50–7.56 (m, 3H, Ar H), 7.67 (d, J = 3.5 Hz, 2H), 7.78–7.82 (m, 3H, Ar H), 7.90 (dd, J = 14.8, 7.8 Hz, 2H), 8.03 (d, J = 7.9 Hz, 2H), 8.10 (s, 1H, CH pyrazole), 9.29 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 81.28 (5-CH furanone), 114.09, 116.23 (2C), 118.53, 119.23 (2C), 123.48, 126.69, 127.80 (2C), 128.88, 129.37 (2C), 130.02, 132.02 (2C), 132.03, 135.66, 137.94, 140.27, 144.12, 144.89, 147.19, 165.42 (C=O); MS (m/z, %): 181.88 (81.84%), 212.46 (100.00%), 236.25 (84.32%), 243.81 (81.69%), 379.41 (67.81%), 472.77 (M+, 34.22%); Anal. Calcd. for C25H18BrN3O2 (472.34): C, 63.57; H, 3.84; Br, 16.92; N, 8.90; O, 6.77; Found: C, 63.51; H, 3.75; Br, 16.83; N, 8.86; O, 6.72.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-((4-methoxyphenyl)amino)furan-2(5H)-one (4j)
From p-anisidine, Yield: 75% as yellow powder; m.p.: 125–127 °C; IR: ν/cm−1: 3417 (NH), 3061 (CH-Ar), 2931, 2836 (CH-aliph), 1710 (C=O), 1598 (C=N), 1450, 1061 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 3.79 (s, 3H, OCH3), 5.83 (d, J = 6.1 Hz, 1H, 5-CH furanone), 7.02 (d, J = 9.0 Hz, 1H, 4-CH furanone), 7.31–7.37 (m, 3H, Ar H), 7.39–7.46 (m, 3H, Ar H), 7.51 (d, J = 7.7 Hz, 2H), 7.55 (dd, J = 16.0, 8.1 Hz, 2H), 7.93 (dd, J = 7.8, 1.6 Hz, 2H), 8.14 (s, 1H, CH pyrazole), 8.65 (d, J = 29.0 Hz, 2H), 9.19 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 55.21 (OCH3), 77.38 (5-CH furanone), 112.60, 114.15, 117.95, 118.57, 119.29 (2C), 122.18, 127.29, 127.47, 127.78, 128.09, 128.59 (2C), 128.72, 129.22, 129.76 (2C), 131.08, 131.27, 132.98, 134.87, 138.62, 152.56, 163.71 (C=O); MS (m/z, %): 43.44 (100.00%), 54.36 (65.24%), 149.32 (71.84%), 338.22 (55.41%), 423.53 (M+, 39.92%); Anal. Calcd. for C26H21N3O3 (423.47): C, 73.74; H, 5.00; N, 9.92; O, 11.33; Found: C, 73.68; H, 4.93; N, 9.85; O, 11.25.
5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-(naphthalen-1-ylamino)furan-2(5H)-one (4k)
From α-naphthylamine, Yield: 78% as brownish powder; m.p.: 129–131 °C; IR: ν/cm−1: 3386 (NH), 3056 (CH-Ar), 2970, 2923 (CH-aliph), 1710 (C=O), 1597 (C=N), 1450, 1059 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 5.13 (d, J = 7.1 Hz, 1H, 5-CH furanone), 7.38 (d, J = 7.4 Hz, 1H, 4-CH furanone), 7.40–7.50 (m, 5H, Ar H), 7.51 (d, J = 7.3 Hz, 4H), 7.57 (d, J = 8.2 Hz, 2H), 7.69 (d, J = 9.2 Hz, 2H), 8.06 (d, J = 7.8 Hz, 2H), 8.15 (s, 1H, CH pyrazole), 8.49 (d, J = 9.2 Hz, 2H), 9.40 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 71.52 (5-CH furanone), 119.01 (2C), 119.47, 119.64, 121.63, 121.84, 124.39, 125.14, 125.20, 126.72, 127.12 (2C), 128.59, 129.38 (3C), 130.06 (2C), 131.18, 132.15, 133.47, 133.94, 134.39, 139.23, 146.17, 150.65, 151.76, 168.60 (C=O); MS (m/z, %): 55.27 (74.65%), 219.87 (100.00%), 249.63 (80.03%), 253.03 (89.02%), 282.93 (96.54%), 443.09 (M+, 35.70%); Anal. Calcd. for C29H21N3O2 (443.51): C, 78.54; H, 4.77; N, 9.47; O, 7.21; Found: C, 78.49; H, 4.70; N, 9.41; O, 7.16.
Ethyl 5-((5-(1,3-diphenyl-1H-pyrazol-4-yl)-2-oxo-2,5-dihydrofuran-3-yl)amino)-1-phenyl-1H-pyrazole-4-carboxylate (4l)
From ethyl 5-amino-1-phenyl-1H-pyrazole-4-carboxylate [53], Yield: 68% as yellow powder; m.p.: 126–128 °C; IR: ν/cm−1: 3395 (NH), 3060 (CH-Ar), 2905, 2864 (CH-aliph), 1751, 1673 (C=O), 1598 (C=N), 1452, 1056 (C–O); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 1.26 (t, J = 7.1 Hz, 3H,CH2CH3), 4.20 (q, J = 7.1 Hz, 2H, CH2CH3), 6.26 (d, J = 6.1 Hz, 1H, 5-CH furanone), 7.44 (d, J = 7.5 Hz, 1H, 4-CH furanone), 7.53 (d, J = 7.7 Hz, 2H), 7.55–7.60 (m, 10H, 9 Ar H and CH pyrazole), 7.72 (s, 1H, CH pyrazole), 7.93 (dd, J = 7.8, 1.6 Hz, 2H), 8.00 (d, J = 7.7 Hz, 2H), 9.32 (s, 1H, NH exchangeable by D2O); 13C NMR (101 MHz, DMSO-d6) δ/ppm: 14.94 (CH3), 59.47 (CH2), 72.11 (5-CH furanone), 118.57, 119.73 (2C), 122.62, 124.09 (2C), 128.02, 128.21, 128.39, 128.92 (2C), 129.03, 129.17 (2C), 129.66, 129.94 (2C), 130.20 (2C), 131.72, 135.31, 138.31, 139.07, 140.64, 150.19, 153.16, 164.04 (C=O), 175.37 (C=O); MS (m/z, %): 196.26 (100.00%), 267.45 (64.33%), 372.24 (46.49%), 502.92 (63.49%), 511.21 (86.90%), 531.98 (M+, 21.69%); Anal. Calcd. for C31H25N5O4 (531.57): C, 70.05; H, 4.74; N, 13.18; O, 12.04; Found: C, 70.00; H, 4.68; N, 13.13; O, 11.98.
Antiviral activity
Cytotoxicity assay
The samples were diluted using Dulbecco’s Modified Eagle’s Medium (DMEM). The test chemicals were dissolved in 10% DMSO in dd H2O. Supplementary to the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) technique, the cytotoxic activity of the extracts was evaluated in Vero E6 cells. The cells were seeded in 96 well-plates (100 µl/well at a density of 3 × 105 cells/ml) and then incubated for 24 h at 37 °C with 5% CO2. Cells were treated in triplicate with different doses of the investigated substances after 24 h. The cell monolayers were washed three times with sterile phosphate buffer saline (PBS) after another 24 h, and then 20 µl of MTT solution, which is a stock solution with a concentration of 5 mg/ml, was added to each well. The wells were then incubated at 37 °C for 4 h prior to medium aspiration. The formazan crystals that had been produced were dissolved in 200 µl of acidified isopropanol (0.04 M HCl in absolute isopropanol = 0.073 ml HCl in 50 ml of isopropanol). The formazan solutions’ absorbance was measured using a multi-well plate reader at a maximum wavelength of 540 nm, with 620 nm serving as a reference wavelength. By plugging the treated cells’ numbers into the following equation, we can calculate the relative cytotoxicity of the drugs. To find the concentration that showed 50% cytotoxicity, the percent cytotoxicity vs. sample concentration plot was generated (TC50).
Plaque reduction assay
In a six-well plate, Vero E6 cells were cultured for 24 h at 37 °C before the experiment was conducted using the Hayden [54] technique. The dilution of the Sever Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) to an approximate concentration of 103 PFU/well was performed. This solution was then combined with the tested compounds at a safe concentration and incubated at 37 °C for 1 h prior to their introduction into the cells. The cell culture plates were rinsed with a growth medium before being inoculated with a virus (100 µl/well) containing the evaluated compounds. Following a 1-h contact time to allow for virus adsorption, 3 ml of DMEM supplemented with 2% agarose and the tested compounds were added to the cell monolayer. The plates were left to solidify and incubated at 37 °C until viral plaques formed (3–4 days). The plates were dyed with 0.1% crystal violet in distilled water after being treated with 10% formalin for two hours. Control wells, which contained Vero E6 cells cultured with an untreated virus, were incorporated into the experiment. After incubation, we counted the plaques and reported the percentage reduction in plaque development compared to the control wells as follows:
where the viral count (untreated) represents the number of viruses present in wells that did not receive any treatment from the compounds. Moreover, the viral count (treated) represents the quantity of viruses present in wells that underwent treatment with the compounds.
DFT studies
All the required density-functional theory (DFT) computations and visualisations [55] are performed using the programs Gaussian-09W and Gauss view-06. Following previously documented procedures [56], all calculations pertaining to this investigation have been performed utilizing the B3LYP functional and the 6-31G(d,p) as the program’s basis set. The basis set is employed to optimize the molecular structures of molecules 4c–e and 4h–j. Additionally, the geometrical parameters, MEP, and FMO orbitals were all constructed using the identical technique’s basis set.
Protein preparation and molecular docking study
The PDB file format was used to construct the structures of the compounds from the output of the Gaussian 09 software. Sourced from the protein data bank (http://www.rcsb.org.pdb), the crystal structures of SARS-CoV-2 main protease (PDB ID: 6Y84) and SARS-CoV-2 Nsp9 RNA binding protein (PDB ID: 6W4B) were used. Molecular docking studies [57,58,59] were conducted using the MOE 2015 software, and the docking technique was carried out as previously reported [60, 61].
Pharmacophore studies
Designing pharmacophores
The methodology for generating pharmacophores, as previously documented [62], is described here. To begin, the MOE 2015.10 software utilized a training set consisting of the chosen SARS-CoV-2 inhibitors using a flexible alignment set. The data is contained inside the flexible alignment output (S: The alignment score of the configuration). Reduced S values could indicate improved alignments. Second, paste the alignment structure with the lowest S value into the MOE window. Utilize the Pharmacophore Query Editor to generate a pharmacophore query for the compounds, including those in the alignment training set. The model that was produced is then evaluated using the Pharmacophore Search on the whole set of test sets 4c–e, and 4h–j. The application subsequently utilizes the Pharmacophore Preprocessor to generate annotations for the conformations of molecules in the test set database, employing the PCH-All (Polarity-Charge-Hydrophobicity) pharmacophore scheme. After modifying the query using the consensus query approach, conduct a database investigation. In the last step, the program gives the rmsd values that represent the degrees of mapping from a specific molecule to a hypothetical model that was built.
Conclusion
The three-component reaction of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (1) with pyruvic acid (2) and aromatic amine derivatives 3a–l in boiling acetic acid proceeded unexpectedly, resulting in 12 derivatives of 3,5-disubstituted furane-2(5H)-one in yields ranging from 68% to 83%. Simplified reaction conditions, an easy work-up approach, and facile separation characterize the procedure employed in the preparation technique. The antiviral efficacy of the newly synthesized compounds against SARS-CoV-2 was evaluated using the MTT assay. Most of the studied compounds exhibited moderate to significant anti-SARS-CoV-2 activity. Also, the plaque reduction assay was used to measure the percentage of inhibition for all the novel compounds. A basis set of DFT/B3LYP/6-31G(d,p) was used for the optimization of 4c–e, and 4h–j. The band gap energy for the examined compounds, which indicates the charge transfer inside the molecules, ranges from 0.14836 to 0.17273 eV, according to the FMO study. Using the MEP map, the electron distribution and surface location of the specified compounds were evaluated. Molecular docking feedback revealed the antiviral activity of the more potent compounds with very low binding energies against SARS-CoV-2 main protease (PDB ID: 6Y84) and Nsp9 RNA binding protein (PDB ID: 6W4B). Models of pharmacophores were ultimately constructed using eleven SARS-CoV-2 inhibitors. The synthesized compounds 4c–e, and 4h–j were shown to have strong and specific inhibitory action against SARS-CoV-2 by 3D pharmacophore virtual screening, demonstrating the utility of these synthetic compounds in medication development.
Data availability
The datasets generated during and/or analyzed during the current study are available at https://www.scidb.cn/en/anonymous/UlJibU0z.
References
Fusetani N, Takahashi M, Matsunaga S (1994) Topsentiasterol sulfates, antimicrobial sterol sulfates possessing novel side chains, from a marine sponge, Topsentia sp. Tetrahedron 50(26):7765–7770
Guo YW, Gavagnin M, Mollo E, Trivellone E, Cimino G (1999) Three new butenolide lipids from the Caribbean gorgonian Pterogorgia anceps. J Nat Prod 62(8):1194–1196
Boalino DM, Connolly JD, McLean S, Reynolds WF, Tinto WF (2003) α-Pyrones and a 2(5H)-furanone from Hyptis pectinata. Phytochemistry 64(7):1303–1307
Reichstein T (1967) Cardenolides as chemical weapons in insects. Cron Chim 15:3–12
Grossmann G, Poncioni M, Bornand M, Jolivet B, Neuburger M, Séquin U (2003) Bioactive butenolides from Streptomyces antibioticus TÜ 99: absolute configurations and synthesis of analogs. Tetrahedron 59(18):3237–3251
Levy LM, Cabrera GM, Wright JE, Seldes AM (2003) 5H-Furan-2-ones from fungal cultures of Aporpium caryae. Phytochemistry 62(2):239–243
Hein SM, Gloer JB, Koster B, Malloch D (2001) Bombardolides: new antifungal and antibacterial γ-lactones from the coprophilous fungus Bombardioidea a nartia. J Nat Prod 64(6):809–812
Pour M, Špulák M, Balšánek V, Kuneš J, Kubanová P, Buchta V (2003) Synthesis and structure–antifungal activity relationships of 3-aryl-5-alkyl-2,5-dihydrofuran-2-ones and their carbanalogues: further refinement of tentative pharmacophore group. Bioorg Med Chem 11(13):2843–2866
Padakanti S, Pal M, Yeleswarapu KR (2003) An improved and practical synthesis of 5,5-dimethyl-3-(2-propoxy)-4-(4-methanesulfonylphenyl)-2-(5H)-furanone (DFP—a selective inhibitor of cyclooxygenase-2). Tetrahedron 59(40):7915–7920
Takahashi S, Kubota A, Nakata T (2002) Total synthesis of muconin. Tetrahedron Lett 43(48):8661–8664
Choudhury A, Jin F, Wang D, Wang Z, Xu G, Nguyen D et al (2003) A concise synthesis of anti-viral agent F-ddA, starting from (S)-dihydro-5-(hydroxymethyl)-2(3H)-furanone. Tetrahedron Lett 44(2):247–250
Kotora M, Negishi EI (1997) Highly efficient and selective procedures for the synthesis of γ-alkylidenebutenolides via palladium-catalyzed ene-yne coupling and palladium- or silver catalyzed lactonization of (Z)-2-en-4-ynoic acids synthesis of rubrolides A, C, D, and E. Synthesis 1997(01):121–128
Xiao Q, Liu Y, Qiu Y, Zhou G, Mao C, Li Z et al (2010) Potent antitumor mimetics of annonaceous acetogenins embedded with an aromatic moiety in the left hydrocarbon chain part. J Med Chem 54(2):525–33
Mao C, Han B, Wang LS, Wang S, Yao ZJ (2011) Modular assembly of cytotoxic acetogenin mimetics by click linkage with nitrogen functionalities. MedChemComm 2(9):918–922
Derbré S, Gil S, Taverna M, Boursier C, Nicolas V, Demey-Thomas E et al (2008) Highly cytotoxic and neurotoxic acetogenins of the Annonaceae: new putative biological targets of squamocin detected by activity-based protein profiling. Bioorg Med Chem Lett 18(21):5741–5744
Abe M, Kubo A, Yamamoto S, Hatoh Y, Murai M, Hattori Y et al (2008) Dynamic function of the spacer region of acetogenins in the inhibition of bovine mitochondrial NADH-ubiquinone oxidoreductase (complex I). Biochemistry 47(23):6260–6266
Kumar A, Singh V, Ghosh S (2012) Butenolide: a novel synthesis and biological activities: a research for novel drug development. LAP LAMBERT Academic Publishing
Jusseau X, Chabaud L, Guillou C (2014) Synthesis of γ-butenolides and α,β-unsaturated γ-butyrolactams by addition of vinylogous nucleophiles to Michael acceptors. Tetrahedron 70(16):2595–2615
Yanai H (2021) Green and catalytic methods for γ-lactone synthesis. Green synthetic approaches for biologically relevant heterocycles. Elsevier, pp 537–615
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M et al (2005) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139(4):1762–1772
Doebner O, Gieseke M (1887) 2) Ueber α-Phenylcinchoninsäure und ihre Homologen. Justus Liebigs Ann Chem 242(3):290–300
Doebner O (1887) Ueber α-Alkylcinchoninsäuren. Ber Dtsch Chem Ges 20(1):277–281
Eftekhari-Sis B, Zirak M (2015) Chemistry of α-oxoesters: a powerful tool for the synthesis of heterocycles. Chem Rev 115(1):151–264
Bhatt HG, Agrawal YK, Patel MJ (2015) Amino- and fluoro-substituted quinoline-4-carboxylic acid derivatives: MWI synthesis, cytotoxic activity, apoptotic DNA fragmentation and molecular docking studies. Med Chem Res 24:1662–1671
Wang LM, Hu L, Chen HJ, Sui YY, Shen W (2009) One-pot synthesis of quinoline-4-carboxylic acid derivatives in water: ytterbium perfluorooctanoate catalyzed Doebner reaction. J Fluorine Chem 130(4):406–409
Borsche W (1908) Über die Synthese α-substituierter Cinchoninsäuren nach Döbner. Ber Dtsch Chem Ges 41(3):3884–3894
Um SJ, Park SH, Park CH, Chang BH, Yoon JH, Sin HS (2003) Synthesis of novel quinolinecarboxamide derivatives with estrogenic activity. Bull Kor Chem Soc 24(5):677–680
Vaughan WR, Peters LR (1953) 2,3-Pyrrolidinediones. I. Preparation and structure. J Org Chem 18(4):382–392
Meyer WL, Vaughan WR (2002) 1,5-Diaryl-2,3-pyrrolidinediones. IX. Reassignment of structure1. J Org Chem 22(12):1560–1565
Mohamed SF, Abd-Elghaffar HS, Amr AEGE, Elnaggar DH, Abou-Amra ES, Hosny HM et al (2023) New poly heterocyclic compounds based on pyrimidine-2-thiones: synthesis, evaluation of putative antiviral agents, DFT calculation, and molecular modeling. J Mol Struct 1291:136083
Mohamed AM, El-Bayaa MN, Elnaggar DH, Abdel-Hafez NA, Mohamed SF, Elsayed MA et al (2023) Novel [1,2,3]triazoles, [1,2,3]triazolo[4,5-d]Pyrimidines, and some of their glycoside derivatives: synthesis and molecular modeling as potential apoptotic antitumor agents. Polycyclic Aromatic Comp 44:1–25
Elnaggar DH, Mohamed AM, Abdel Hafez NA, Azab ME, Elasasy ME, Awad HM et al (2022) Antiproliferative activity of some newly synthesized substituted nicotinamides candidates using pyridine-2(1H) thione derivatives as synthon. ACS Omega 7(12):10304–10316
Mohamed AM, Abdelwahab M, Abde lHafez NA, Mahmoud SF, El-Bayaa M, El-Kady DS et al (2022) Synthesis, cytotoxic activity and molecular modelling of novel [1,2,3]triazolo[4,5-d] pyrimidine compounds, their glycoside derivatives and acyclic analogs. Egypt J Chem 65(1):645–656
Sridhar R, Srinivas B, Kumar VP, Reddy VP, Kumar AV, Rao KR (2008) Novel aqueous phase supramolecular synthesis of 3-pyrrolylindolin-2-ones and pyrrolylindeno[1,2-b]quinoxalines. Adv Synth Catal 350(10):1489–1492
Bassetti M, D’Annibale A, Fanfoni A, Minissi F (2005) Synthesis of α,β-unsaturated 4,5-disubstituted γ-Lactones via ring-closing metathesis catalyzed by the first-generation grubbs’ catalyst. Org Lett 7(9):1805–1808
Ather AQ, Tahir MN, Khan MA, Mehmood K, Chaudhry F (2010) 1,3-Diphenyl-1H-pyrazole-4-carbaldehyde. Acta Crystallogr Sect E Struct Rep Online 66(12):o3170–o3170
Singh K, Ralhan S, Sharma PK, Dhawan SN (2005) Vilsmeier-Haack reaction on hydrazones: a convenient synthesis of 4-formylpyrazoles. J Chem Res 2005(5):316–318
Karnan M, Balachandran V, Murugan M, Murali MK (2016) Scaled quantum chemical calculations and FTIR, FT-Raman spectra, NBO, thermodynamical behavior, HOMO-LUMO and electronic structure calculatuions on 4-(dimethylamine) benzophenone. Elixir Vib Spectrosc 91:38114–38124
Chebanov VA, Gura KA, Desenko SM (2010) Aminoazoles as key reagents in multicomponent heterocyclizations. Synthesis of heterocycles via multicomponent reactions I. Springer, pp 41–84
Sakhno YI, Desenko SM, Shishkina SV, Shishkin OV, Sysoyev DO, Groth U et al (2008) Multicomponent cyclocondensation reactions of aminoazoles, arylpyruvic acids and aldehydes with controlled chemoselectivity. Tetrahedron 64(49):11041–11049
Sakhno YI, Shishkina SV, Shishkin OV, Musatov VI, Vashchenko EV, Desenko SM, Chebanov VA (2010) Diversity oriented heterocyclizations of pyruvic acids, aldehydes and 5-amino-N-aryl-1H-pyrazole-4-carboxamides: catalytic and temperature control of chemoselectivity. Mol Divers 14:523–531
Chebanov VA, Saraev VE, Desenko SM, Chernenko VN, Knyazeva IV, Groth U et al (2008) Tuning of chemo- and regioselectivities in multicomponent condensations of 5-aminopyrazoles, dimedone, and aldehydes. J Org Chem 73(13):5110–5118
Muravyova EA, Shishkina SV, Musatov VI, Knyazeva IV, Shishkin OV, Desenko SM, Chebanov VA (2009) Chemoselectivity of multicomponent condensations of barbituric acids, 5-aminopyrazoles, and aldehydes. Synthesis 2009(08):1375–1385
Sakhno YI, Desenko SM, Shishkina SV, Shishkin OV, Musatov VI, Chebanov VA (2011) Unusual direction of cyclocondensation of 1-(4-chlorophenyl)-3,5-diamino-1,2,4-triazole, pyruvic acid, and aldehydes. Synthesis 2011:1120–1124
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 178:104787
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Fukui K (1982) Role of frontier orbitals in chemical reactions. Science 218(4574):747–754
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516
Saravanan S, Balachandran V (2014) Quantum chemical studies, natural bond orbital analysis and thermodynamic function of 2,5-dichlorophenylisocyanate. Spectrochim Acta Part A Mol Biomol Spectrosc 120:351–364
Deshpande RR, Tiwari AP, Nyayanit N, Modak M (2020) In silico molecular docking analysis for repurposing therapeutics against multiple proteins from SARS-CoV-2. Eur J Pharmacol 886:173430
Mason JS, Good AC, Martin EJ (2001) 3-D pharmacophores in drug discovery. Curr Pharm Des 7(7):567–597
El-Tombary AA, Abdel-Ghany YS, Belal AS, Shams El-Dine SA, Soliman FS (2011) Synthesis of some substituted furan-2(5H)-ones and derived quinoxalinones as potential anti-microbial and anti-cancer agents. Med Chem Res 20:865–876
Hassan AY, Kadh MS, Saleh NM, Abou-Amra ES (2019) A novel synthesis of fused pyrazolopyrimidine: pyrazolo-triazolo-pyrimidine for anticancer evaluation. Int J Adv Res 3(8):55–59
Hayden FG, Cote KM, Douglas RG Jr (1980) Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob Agents Chemother 17(5):865–870
Christenholz CL, Obenchain DA, Peebles RA, Peebles SA (2014) Rotational spectroscopic studies of C-H··· F interactions in the vinyl fluoride··· difluoromethane complex. J Phys Chem A 118(9):1610–1616
Hassan AY, Shabaan SN, El-Sebaey SA, Abou-Amra ES (2023) Synthesis of pyrido-annelated [1,2,4,5]tetrazines, [1,2,4]triazepine, and [1,2,4,5]tetrazepines for anticancer, DFT, and molecular docking studies. Sci Rep 13(1):5585
Mathada BS, Basha NJ, Javeed M, Karunakar P, Venkatesulu A, Erappa K, Varsha A (2024) Novel pyrimidines as COX-2 selective inhibitors: synthesis, DFT analysis, molecular docking and dynamic simulation studies. J Biomol Struct Dyn 42(4):1751–1764
Mathada BS, Basha NJ, Karunakar P, Periyasamy G, Somappa SB, Javeed M, Vanishree S (2023) Investigation of embelin synthetic hybrids as potential COVID-19 and COX inhibitors: synthesis, spectral analysis, DFT calculations and molecular docking studies. J Mol Struct 1273:134356
Horchani M, Heise NV, Csuk R, Ben Jannet H, Harrath AH, Romdhane A (2022) Synthesis and in silico docking study towards M-pro of novel heterocyclic compounds derived from pyrazolopyrimidinone as putative SARS-CoV-2 inhibitors. Molecules 27(16):5303
Hassan AY, Abou-Amra ES, El-Sebaey SA (2023) Design and synthesis of new series of chiral pyrimidine and purine analogs as COX-2 inhibitors: anticancer screening, molecular modeling, and in silico studies. J Mol Struct 1278:134930
Alghamdi HA, Attique SA, Yan W, Arooj A, Albulym O, Zhu D et al (2021) Repurposing the inhibitors of COVID-19 key proteins through molecular docking approach. Process Biochem 110:216–222
Ellithy SA, Abdel-Rahman A, Abou-Amra ES, Hassan AA (2023) Glycosyl thiourea: synthesis, cyclization, reaction, molecular docking, and evaluation as potential acetylcholinesterase inhibitors. Egypt J Chem 66:1759–1777
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
All authors were involved in the synthesis, structure elucidation, and writing-original draft preparation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsisi, D.M., Mohamed, A.M., Seadawy, M.G. et al. One pot multi-component synthesis of novel functionalized pyrazolo furan-2(5H)-one derivatives: in vitro, DFT, molecular docking, and pharmacophore studies, as coronavirus inhibitors. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10885-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10885-x