Abstract
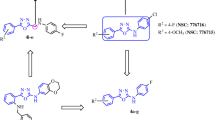
Many human cancers have been associated with the deregulation of the mesenchymal-epithelial transition factor tyrosine kinase (MET) receptor, a promising drug target for anticancer drug discovery. Herein, we report the discovery of a novel structure of potent chalcone-based derivatives type II c-Met inhibitors which are comparable to Foretinib (IC50 = 14 nM) as a potent reference drug. Based on our design strategy, we also expected an anti-tubulin activity for the compounds. However, the weak inhibitory effects on microtubules were confirmed by cell cycle analyses implicated that the observed cytotoxicity against HeLa cells probably was not derived from tubulin inhibition. Compounds 14q and 14k with IC50 values of 24 nM and 45 nM, respectively, demonstrated favorable inhibition of MET kinase activity, and desirable bonding interactions in the ligand-MET enzyme complex stability in molecular docking studies.







Similar content being viewed by others
References:
Eder JP et al (2009) Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15(7):2207–2214. https://doi.org/10.1158/1078-0432
Goetsch L, Caussanel V, Corvaia N (2013) Biological significance and targeting of c-Met tyrosine kinase receptor in cancer. Front Biosci 18(2):454–473
O’toole, J.M., et al (2006) Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res 66(18):9162–9170
Organ SL, Tsao M-S (2011) An overview of the c-MET signaling pathway. Ther Adv Med Oncol. https://doi.org/10.1177/1758834011422556
Zhang Y et al (2018) Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer 17(1):1–14. https://doi.org/10.1186/s12943-018-0796-y
Liang D et al (2022) Discovery of small-molecule fluorescent probes for C-Met. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2022.114114
Parikh PK, Ghate MD (2018) Recent advances in the discovery of small molecule c-Met Kinase inhibitors. Eur J Med Chem 143:1103–1138. https://doi.org/10.1016/j.ejmech.2017.08.044
Birchmeier C et al (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4(12):915–925. https://doi.org/10.1038/nrm1261
Mohyeldin MM et al (2016) Novel c-Met inhibitory olive secoiridoid semisynthetic analogs for the control of invasive breast cancer. Eur J Med Chem 118:299–315. https://doi.org/10.1016/j.ejmech.2016.04.043
Yuan H et al (2018) Discovery, optimization and biological evaluation for novel c-Met kinase inhibitors. Eur J Med Chem 143:491–502
Dharmawardana PG, Giubellino A, Bottaro DP (2004) Hereditary papillary renal carcinoma type I. Curr Mol Med 4(8):855–868. https://doi.org/10.2174/1566524043359674
Wang Z et al (2020) Design, synthesis and biological evaluation of novel 4-phenoxypyridine based 3-oxo-3, 4-dihydroquinoxaline-2-carboxamide derivatives as potential c-Met kinase inhibitors. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2020.104371
El-Wakil MH, Teleb M (2021) Transforming Type II to Type I c-Met kinase inhibitors via combined scaffold hopping and structure-guided synthesis of new series of 1, 3, 4-thiadiazolo [2, 3-c]-1, 2, 4-triazin-4-one derivatives. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105304
Ibrahim HS et al (2019) Synthesis and biological evaluation of some novel thiobenzimidazole derivatives as anti-renal cancer agents through inhibition of c-MET kinase. Bioorg Chem 85:337–348. https://doi.org/10.1016/j.bioorg.2019.01.006
Puccini A et al (2019) Safety and tolerability of c-MET inhibitors in cancer. Drug Saf 42:211–233. https://doi.org/10.1007/s40264-018-0780-x
Mo H-N, Liu P (2017) Targeting MET in cancer therapy. Chronic Dis Transl Med. 3(03):148–153
Katayama R et al (2013) Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res 73(10):3087–3096. https://doi.org/10.1158/0008-5472.CAN-12-3256
Best J et al (2017) Tivantinib for the treatment of hepatocellular carcinoma. Expert Opin Pharmacother 18(7):727–733. https://doi.org/10.1080/14656566.2017.1316376
Rimassa L et al (2018) Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 19(5):682–693. https://doi.org/10.1016/S1470-2045(18)30146-3
Adjei AA, Schwartz B, Garmey E (2011) Early clinical development of ARQ 197, a Selective, Non–ATP-competitive inhibitor targeting MET Tyrosine kinase for the treatment of advanced cancers. Oncologist 16(6):788–799. https://doi.org/10.1634/theoncologist.2010-0380
Goldman JW et al (2012) Phase 1 dose-escalation trial evaluating the combination of the selective MET (mesenchymal-epithelial transition factor) inhibitor tivantinib (ARQ 197) plus erlotinib. Cancer 118(23):5903–5911. https://doi.org/10.1002/cncr.27575
Shuai W et al (2021) Recent progress on tubulin inhibitors with dual targeting capabilities for cancer therapy. J Med Chem 64(12):7963–7990. https://doi.org/10.1021/acs.jmedchem.1c00100
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9(10):790–803. https://doi.org/10.1038/nrd3253
Yang C-PH, Horwitz SB (2017) Taxol®: the first microtubule stabilizing agent. Int J Mol Sci 18(8):1733. https://doi.org/10.3390/ijms18081733
Campiani G et al (2022) Design and synthesis of multifunctional microtubule targeting agents endowed with dual pro-apoptotic and anti-autophagic efficacy. Eur J Med Chem 235:114274. https://doi.org/10.1016/j.ejmech.2022.114274
Steinmetz MO, Prota AE (2018) Microtubule-targeting agents: strategies to hijack the cytoskeleton. Trends Cell Biol 28(10):776–792. https://doi.org/10.1016/j.tcb.2018.05.001
Liang T et al (2022) Combination of microtubule targeting agents with other antineoplastics for cancer treatment. Biochim Biophys Acta Rev Cancer. https://doi.org/10.1016/j.bbcan.2022.188777
Serpico AF, Visconti R, Grieco D (2020) Exploiting immune-dependent effects of microtubule-targeting agents to improve efficacy and tolerability of cancer treatment. Cell Death Dis 11(5):1–7. https://doi.org/10.1038/s41419-020-2567-0
Cao Y-N et al (2018) Recent advances in microtubule-stabilizing agents. Eur J Med Chem 143:806–828
Kavallaris M (2010) Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer 10(3):194–204. https://doi.org/10.1038/nrc2803
Zhu H et al (2020) Design, synthesis and biological evaluation of vinyl selenone derivatives as novel microtubule polymerization inhibitors. Eur J Med Chem 207:112716. https://doi.org/10.1016/j.ejmech.2020.112716
Rosini M (2014) Polypharmacology: the rise of multitarget drugs over combination therapies. Future Med Chem 6(5):485–487. https://doi.org/10.4155/fmc.14.25
Talevi A (2015) Multi-target pharmacology: possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front pharmacol 6:205. https://doi.org/10.3389/fphar.2015.00205
L Bolognesi, M., Polypharmacology in a single drug: multitarget drugs. Curr. Med. Chem., 2013. 20(13): p. 1639–1645
Arnst KE et al (2019) Current advances of tubulin inhibitors as dual acting small molecules for cancer therapy. Med Res Rev 39(4):1398–1426. https://doi.org/10.1002/med.21568
Tanabe K (2017) Microtubule depolymerization by kinase inhibitors: unexpected findings of dual inhibitors. Int J Mol Sci 18(12):2508. https://doi.org/10.3390/ijms18122508
Tian C et al (2022) Discovery of (2-(pyrrolidin-1-yl) thieno [3, 2-d] pyrimidin-4-yl)(3, 4, 5-trimethoxyphenyl) methanone as a novel potent tubulin depolymerizing and vascular disrupting agent. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2022.114466
Kim DY et al (2006) Design and biological evaluation of novel tubulin inhibitors as antimitotic agents using a pharmacophore binding model with tubulin. J Med Chem 49(19):5664–5670. https://doi.org/10.1021/jm050761i
Bellon SF et al (2008) c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J Biol Chem 283(5):2675–2683. https://doi.org/10.1074/jbc.M705774200
D’Angelo ND et al (2008) Design, synthesis, and biological evaluation of potent c-Met inhibitors. J Med Chem 51(18):5766–5779. https://doi.org/10.1021/jm8006189
Zhu W et al (2016) Synthesis, and docking studies of phenylpyrimidine-carboxamide derivatives bearing 1H-pyrrolo [2, 3-b] pyridine moiety as c-Met inhibitors. Bioorg Med Chem 24(8):1749–1756. https://doi.org/10.1016/j.bmc.2016.02.046
Nakagawa T et al (2010) E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci 101(1):210–215. https://doi.org/10.1111/j.1349-7006.2009.01343.x
Kataoka Y et al (2012) Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Invest New Drugs 30(4):1352–1360. https://doi.org/10.1007/s10637-011-9699-0
Koziel R et al (2010) Ciprofloxacin inhibits proliferation and promotes generation of aneuploidy in Jurkat cells. J Physiol Pharmacol 61(2):233
Aranha O et al (2003) Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol 22(4):787–794. https://doi.org/10.3892/ijo.22.4.787
Toolabi M et al (2022) Synthesis of novel 2-acetamide-5-phenylthio-1, 3, 4-thiadiazole-containing phenyl urea derivatives as potential VEGFR-2 inhibitors. Arch Pharm 355(3):2100397. https://doi.org/10.1002/ardp.202100397
Mohammadian E et al (2022) Thienopyrimidine-based agents bearing diphenylurea Design, synthesis, and evaluation of antiproliferative and antiangiogenic activity. Arch Pharm. https://doi.org/10.1002/ardp.202200349
Wang B et al (2019) Anti-tumor activity evaluation of novel tubulin and HDAC dual-targeting inhibitors. Bioorg Med Chem Lett 29(18):2638–2645. https://doi.org/10.1016/j.bmcl.2019.07.045
Li L et al (2018) Pt (IV) prodrugs containing microtubule inhibitors displayed potent antitumor activity and ability to overcome cisplatin resistance. Eur J Med Chem 156:666–679. https://doi.org/10.1016/j.ejmech.2018.07.016
Acknowledgements
This work was supported and funded by a grant from the Tehran University of Medical Sciences grant no. 55283. Moreover, we are truly grateful to the University of Tsukuba for conducting the biological assays.
Funding
This article was funded by Tehran University of Medical Sciences, 55283.
Author information
Authors and Affiliations
Contributions
All authors had the same contribution in this manuscript.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salarinejad, S., Seyfi, S., Hayashi, S. et al. Design, synthesis, and biological evaluation of new biaryl derivatives of cycloalkyl diacetamide bearing chalcone moiety as type II c-MET kinase inhibitors. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10807-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10807-x