Abstract
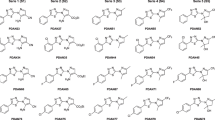
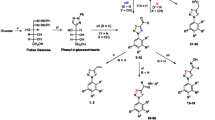
Tropical diseases, such as African trypanosomiasis, by their nature and prevalence lack the necessary urgency regarding drug development, despite the increasing need for novel, structurally diverse antitrypanosomal drugs, using different mechanisms of action that would improve drug efficacy and safety. Traditionally antibacterial agents, the fluoroquinolones, reportedly possess in vitro trypanocidal activities against Trypanosoma brucei organisms. During our research, the fluroquinolone, ciprofloxacin (1), and its analogs (2–24) were tested against bloodstream forms of T. brucei brucei, T. b. gambiense, T. b. rhodesiense, T. evansi, T. equiperdum, and T. congolense and Madin-Darby bovine kidney cells (cytotoxicity). Ciprofloxacin [CPX (1)] demonstrated selective trypanocidal activity against T. congolense (IC50 7.79 µM; SI 39.6), whereas the CPX derivatives (2–10) showed weak selective activity (25 < IC50 < 65 µM; 2 < SI < 4). Selectivity and activity of the CPX and 1,2,3-triazole (TZ) hybrids (11–24) were governed by their chemical functionality at C-3 (carboxylic acid, or 4-methylpiperazinyl amide) and their electronic effect (electron-donating or electron-withdrawing para-benzyl substituent), respectively. Trypanocidal hits in the micromolar range were identified against bloodstream forms of T. congolense [CPX (1); CPX amide derivatives 18: IC50 8.95 µM; SI 16.84; 22: IC50 5.42 µM; SI 25.2] and against T. brucei rhodesiense (CPX acid derivative 13: IC50 4.51 µM; SI 10.2), demonstrating more selectivity toward trypanosomes than mammalian cells. Hence, the trypanocidal hit compound 22 may be optimized by retaining the 4-methylpiperazine amide functional group (C-3) and the TZ moiety at position N-15 and introducing other electron-withdrawing ortho-, meta-, and/or para-substituents on the aryl ring in an effort to improve the pharmacokinetic properties and increase the trypanocidal activity.
Graphical abstract
Structure–activity relationships of ciprofloxacin-1,2,3-triazole hybrids were governed by the chemical functionality at C-3 and electronic effect.


Similar content being viewed by others
Data availability
Data will be made available upon request.
Materials availability
Samples of the compounds are available from David. D. N’Da.
References
Altamura F, Rajesh R, Catta-Preta CM, Moretti NS, Cestari I (2022) The current drug discovery landscape for trypanosomiasis and leishmaniasis: challenges and strategies to identify drug targets. Drug Dev Res 83(2):225–252. https://doi.org/10.1002/ddr.21664
Brun R, Blum J, Chappuis F, Burri C (2010) Human African trypanosomiasis. Lancet 375(9709):148–159. https://doi.org/10.1016/S0140-6736(09)60829-1
Büscher P, Cecchi G, Jamonneau V, Priotto G (2017) Human African trypanosomiasis. Lancet 390(10110):2397–2409. https://doi.org/10.1016/S0140-6736(17)31510-6
Cayla M, Rojas F, Silvester E, Venter F, Matthews KR (2019) African trypanosomes. Parasit Vectors 12(1):1–8. https://doi.org/10.1186/s13071-019-3355-5
Desquesnes M, Gonzatti M, Sazmand A, Thévenon S, Bossard G, Boulangé A, Gimonneau G, Truc P, Herder S, Ravel S (2022) A review on the diagnosis of animal trypanosomoses. Parasit Vectors 15(1):1–24. https://doi.org/10.1186/s13071-022-05190-1
Giordani F, Morrison LJ, Rowan TG, De Koning HP, Barrett MP (2016) The animal trypanosomiases and their chemotherapy: a review. Parasitology 143(14):1862–1889. https://doi.org/10.1017/S0031182016001268
Venturelli A, Tagliazucchi L, Lima C, Venuti F, Malpezzi G, Magoulas GE, Santarem N, Calogeropoulou T, Cordeiro-da-Silva A, Costi MP (2022) Current treatments to control African trypanosomiasis and one health perspective. Microorganisms 10(7):1298. https://doi.org/10.3390/microorganisms10071298
Simarro PP, Cecchi G, Paone M, Franco JR, Diarra A, Ruiz JA, Fèvre EM, Courtin F, Mattioli RC, Jannin JG (2010) The atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. Int J Health Geogr 9(1):1–18. https://doi.org/10.1186/1476-072X-9-57
Peacock L, Cook S, Ferris V, Bailey M, Gibson W (2012) The life cycle of Trypanosoma (nannomonas) congolense in the tsetse fly. Parasit Vectors 5(1):1–13. https://doi.org/10.1186/1756-3305-5-109
Desquesnes M, Holzmuller P, Lai DH, Dargantes A, Lun ZR, Jittaplapong S (2013) Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res Int. https://doi.org/10.1155/2013/194176
WHO. Trypanosomiasis, human African (sleeping sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness). Accessed on 23 Nov 2022
Kasozi KI, MacLeod ET, Ntulume I, Welburn SC (2022) An update on African trypanocide pharmaceutics and resistance. Front Vet Sci. https://doi.org/10.3389/fvets.2022.828111
Horn D (2014) Antigenic variation in African trypanosomes. Mol Biochem Parasitol 195(2):123–129. https://doi.org/10.1101/cshperspect.a025320
Field MC, Horn D, Fairlamb AH, Ferguson MA, Gray DW, Read KD, De Rycker M, Torrie LS, Wyatt PG, Wyllie S (2017) Anti-trypanosomatid drug discovery: an ongoing challenge and a continuing need. Nat Rev Microbiol 15(4):217–231. https://doi.org/10.1038/nrmicro.2016.193
Kourbeli V, Chontzopoulou E, Moschovou K, Pavlos D, Mavromoustakos T, Papanastasiou IP (2021) An overview on target-based drug design against kinetoplastid protozoan infections: human African trypanosomiasis, chagas disease and leishmaniases. Molecules 26(15):4629. https://doi.org/10.3390/molecules26154629
Imran M, Khan SA, Alshammari MK, Alqahtani AM, Alanazi TA, Kamal M, Jawaid T, Ghoneim MM, Alshehri S, Shakeel F (2022) Discovery, development, inventions and patent review of fexinidazole: the first all-oral therapy for human African trypanosomiasis. Pharmaceuticals 15(2):128. https://doi.org/10.3390/ph15020128
Sokolova AY, Wyllie S, Patterson S, Oza SL, Read KD, Fairlamb AH (2010) Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob Agents Chemother 54(7):2893–2900. https://doi.org/10.1128/AAC.00332-10
Trouiller P, Olliaro PL (1999) Drug development output: what proportion for tropical diseases? Lancet 354(9173):164. https://doi.org/10.1016/S0140-6736(05)75299-5
Pink R, Hudson A, Mouriès MA, Bendig M (2005) Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discovery 4(9):727–740. https://doi.org/10.1038/nrd1824
Guha R (2013) On exploring structure-activity relationships. In Silico Models Drug Discov. https://doi.org/10.1007/978-1-62703-342-8_6
Keiser J, Burri C (2001) Evaluation of quinolone derivatives for antitrypanosomal activity. Tropical Med Int Health 6(5):369–389. https://doi.org/10.1046/j.1365-3156.2001.00713.x
King DE, Malone R, Lilley SH (2000) New classification and update on the quinolone antibiotics. Am Fam Physician 61(9):2741–2748
Sharma PC, Jain A, Jain S, Pahwa R, Yar MS (2010) Ciprofloxacin: review on developments in synthetic, analytical, and medicinal aspects. J Enzyme Inhib Med Chem 25(4):577–589. https://doi.org/10.3109/14756360903373350
Dalhoff A (2015) Antiviral, antifungal, and antiparasitic activities of fluoroquinolones optimized for treatment of bacterial infections: a puzzling paradox or a logical consequence of their mode of action? Eur J Clin Microbiol Infect Dis 34(4):661–668. https://doi.org/10.1007/s10096-014-2296-3
Betbeder D, Hutchison D, Baltz T, Cros S (1988) Trypanocidal and antitumor activities of nalidixic and oxolinic acid derivatives. Med Sci Res 16:141–142
Hiltensperger G, Hecht N, Kaiser M, Rybak JC, Hoerst A, Dannenbauer N, Müller-Buschbaum K, Bruhn H, Esch H, Lehmann L (2016) Quinolone amides as antitrypanosomal lead compounds with in vivo activity. Antimicrob Agents Chemother 60(8):4442–4452. https://doi.org/10.1128/AAC.01757-15
Nenortas E, Kulikowicz T, Burri C, Shapiro TA (2003) Antitrypanosomal activities of fluoroquinolones with pyrrolidinyl substitutions. Antimicrob Agents Chemother 47(9):3015–3017. https://doi.org/10.1128/AAC.47.9.3015-3017.2003
Tang SC, Shapiro TA (2010) Newly identified antibacterial compounds are topoisomerase poisons in African trypanosomes. Antimicrob Agents Chemother 54(2):620–626. https://doi.org/10.1128/AAC.01025-09
Nenortas E, Burri C, Shapiro TA (1999) Antitrypanosomal activity of fluoroquinolones. Antimicrob Agents Chemother 43(8):2066–2068. https://doi.org/10.1128/AAC.43.8.2066
Hooper DC, Jacoby GA (2016) Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med 6(9):a025320
Balaña-Fouce R, Álvarez-Velilla R, Fernández-Prada C, García-Estrada C, Reguera RM (2014) Trypanosomatids topoisomerase re-visited: new structural findings and role in drug discovery. Int J Parasitol Drugs Drug Resist 4(3):326–337. https://doi.org/10.1016/j.ijpddr.2014.07.006
Andersson MI, MacGowan AP (2003) Development of the quinolones. J Antimicrob Chemother 51(suppl_1):1–11. https://doi.org/10.1093/jac/dkg212
Ma X, Zhou W, Brun R (2009) Synthesis, in vitro antitrypanosomal and antibacterial activity of phenoxy, phenylthio or benzyloxy substituted quinolones. Bioorg Med Chem Lett 19(3):986–989. https://doi.org/10.1016/j.bmcl.2008.11.078
Martinez M, McDermott P, Walker R (2006) Pharmacology of the fluoroquinolones: a perspective for the use in domestic animals. Vet J 172(1):10–28
Chu DT, Fernandes PB (1991) Recent developments in the field of quinolone antibacterial agents. Adv Drug Res 21:39–144. https://doi.org/10.1016/B978-0-12-013321-5.50007-2
Sharma PC, Jain A, Jain S (2009) Fluoroquinolone antibacterials: a review on chemistry, microbiology and therapeutic prospects. Acta Pol Pharm 66(6):587–604
Cilliers P, Seldon R, Smit FJ, Aucamp J, Jordaan A, Warner DF, N’Da DD (2019) Design, synthesis, and antimycobacterial activity of novel ciprofloxacin derivatives. Chem Biol Drug Des 94(2):1518–1536. https://doi.org/10.1111/cbdd.13534
Meunier B (2008) Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res 41(1):69–77. https://doi.org/10.1016/j.tvjl.2005.07.010
Ali AA (2020) 1, 2, 3-triazoles: synthesis and biological application. IntechOpen. https://doi.org/10.5772/intechopen.92692
Kharb R, Sharma PC, Yar MS (2011) Pharmacological significance of triazole scaffold. J Enzyme Inhib Med Chem 26(1):1–21. https://doi.org/10.3109/14756360903524304
Tarawneh AH, Al-Momani L, León F, Jain SK, Gadetskaya AV, Abu-Orabi ST, Tekwani BL, Cutler SJ (2018) Evaluation of triazole and isoxazole derivatives as potential anti-infective agents. Med Chem Res 27(4):1269–1275. https://doi.org/10.1007/s00044-018-2146-4
Lepesheva GI, Waterman MR (2011) Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr Top Med Chem 11(16):2060–2071. https://doi.org/10.2174/156802611796575902
Batiha GES, Tayebwa DS, Beshbishy AM, N’Da DD, Yokoyama N, Igarashi I (2020) Inhibitory effects of novel ciprofloxacin derivatives on the growth of four Babesia species and Theileria equi. Parasitol Res 119(9):3061–3073. https://doi.org/10.1007/s00436-020-06796-z
Suganuma K, Allamanda P, Hakimi H, Zhou M, Angeles JM, Kawazu SI, Inoue N (2014) Establishment of ATP-based luciferase viability assay in 96-well plate for Trypanosoma congolense. J Vet Med Sci. https://doi.org/10.1292/jvms.14-0273
Suganuma K, Yamasaki S, Molefe NI, Musinguzi PS, Davaasuren B, Mossaad E, Narantsatsral S, Battur B, Battsetseg B, Inoue N (2017) The establishment of in vitro culture and drug screening systems for a newly isolated strain of Trypanosoma equiperdum. Int J Parasitol Drugs Drug Resist 7(2):200–205. https://doi.org/10.1016/j.ijpddr.2017.04.002
Munsimbwe L, Seetsi A, Namangala B, N’Da DD, Inoue N, Suganuma K (2021) In vitro and in vivo trypanocidal efficacy of synthesized nitrofurantoin analogs. Molecules 26(11):3372. https://doi.org/10.3390/molecules26113372
Katsuno K, Burrows JN, Duncan K, Van Huijsduijnen RH, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby B (2015) Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 14(11):751–758. https://doi.org/10.1038/nrd4683
Nwaka S, Hudson A (2006) Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov 5(11):941–955. https://doi.org/10.1038/nrd2144
Adewusi EA, Steenkamp P, Fouche G, Steenkamp V (2013) Isolation of cycloeucalenol from Boophone disticha and evaluation of its cytotoxicity. Nat Prod Commun 8(9):906. https://doi.org/10.1177/1934578X13008009
Fu J, Chen H, Soroka DN, Warin RF, Sang S (2014) Cysteine-conjugated metabolites of ginger components, shogaols, induce apoptosis through oxidative stress-mediated p53 pathway in human colon cancer cells. J Agric Food Chem 62(20):4632–4642. https://doi.org/10.1021/jf501351r
Liu S, Su M, Song SJ, Jung JH (2017) Marine-derived Penicillium species as producers of cytotoxic metabolites. Mar Drugs 15(10):329. https://doi.org/10.3390/md15100329
Finiuk N, Hreniuh V, Ostapiuk YV, Matiychuk V, Frolov D, Obushak M, Stoika R, Babsky A (2017) Antineoplastic activity of novel thiazole derivatives. Biopolym Cell 33(2):135–146. https://doi.org/10.7124/bc.00094B
Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J (2014) Clinical development success rates for investigational drugs. Nat Biotechnol 32(1):40–51. https://doi.org/10.1038/nbt.2786
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23(1–3):3–25. https://doi.org/10.1016/S0169-409X(96)00423-1
Talevi A (2022) The ADME Encyclopedia: A Comprehensive Guide on Biopharmacy and Pharmacokinetics. Springer, Cham, pp 725–732. https://doi.org/10.1007/978-3-030-84860-6_11
MSD Veterinary Manual. Quinolones, Including Fluoroquinolones, Use in Animals. https://www.msdvetmanual.com/pharmacology/antibacterial-agents/quinolones,-including-fluoroquinolones,-use-in-animals. Accessed 20 Jun 2023
Pacheco MC, Purser S, Gouverneur V (2008) The chemistry of propargylic and allylic fluorides. Chem Rev 108(6):1943–1981. https://doi.org/10.1021/cr068410e
Purser S, Moore PR, Swallow S, Gouverneur V (2008) Fluorine in medicinal chemistry. Chem Soc Rev 37(2):320–330. https://doi.org/10.1039/B610213C
Testa B, Krämer SD (2007) The biochemistry of drug metabolism: an introduction: part 2: redox reactions and their enzymes. Chem Biodivers 4(3):257–405. https://doi.org/10.1002/cbdv.200790032
Hollenberg PF (2002) Characteristics and common properties of inhibitors, inducers, and activators of CYP enzymes. Drug Metab Rev 34(1–2):17–35. https://doi.org/10.1081/DMR-120001387
Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R, Abraham S, Habet SA, Baweja RK, Burckart GJ (2008) New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol 48(6):662–670. https://doi.org/10.1177/0091270007312153
Lejon V, Bentivoglio M, Franco JR (2013) Human African trypanosomiasis. Handb Clin Neurol 114:169–181. https://doi.org/10.1016/B978-0-444-53490-3.00011-X
Green HP, Portela MD, Jean EN, Lugli EB, Raper J (2003) Evidence for a Trypanosoma brucei lipoprotein scavenger receptor. J Biol Chem 278(1):422–427. https://doi.org/10.1074/jbc.M207215200
Nok AJ, Nock IH, Bonire JJ (2003) The cholesterol pathway of Trypanosoma congolense could be a target for triphenyltinsalicylate and triphenylsiliconsalicylate inhibition. Appl Organomet Chem 17(1):17–22. https://doi.org/10.1002/aoc.368
Rahman A, O’Sullivan P, Rozas I (2019) Recent developments in compounds acting in the DNA minor groove. Medchemcomm 10(1):26–40. https://doi.org/10.1039/C8MD00425K
Fersing C, Boudot C, Castera-Ducros C, Pinault É, Hutter S, Paoli-Lombardo R, Primas N, Pedron J, Séguy L, Bourgeade-Delmas S, Sournia-Saquet A, Stigliani J, Brossas J, Paris L, Valentin A, Wyllie S, Fairlamb AH, Boutet-Robinet E, Corvaisier S, Since M, Malzert-Fréon A, Destere A, Mazier D, Rathelot P, Courtioux B, Azas N, Verhaeghe P, Vanelle P (2020) 8-Alkynyl-3-nitroimidazopyridines display potent antitrypanosomal activity against both T. b. brucei and cruzi. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2020.112558
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7(1):1–13. https://doi.org/10.1038/srep42717
Hirumi H, Hirumi K (1991) In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology 102(2):225–236. https://doi.org/10.1017/S0031182000062533
Acknowledgements
The authors would like to thank the Japan Society for the Promotion of Science (JSPS) and Ohyama Health Foundation, Inc. for providing financial assistance.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Number 16K18793 [Grants-in-Aid for Young Scientists (B)], Grant Number 148781 [NRF-JSPS Bilateral Joint Research Program], and Ohyama Health Foundation, Inc. The funders had no role in the study design, data collection, and interpretation, nor the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
KS and DDN contributed to conceptualization, funding, and bio resources; KS contributed to investigation; HDJR contributed to writing manuscript; and KS, DDN, and HDJR contributed to review and editing . All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, in the writing of the manuscript, nor in the decision to publish the results.
Ethical approval
Not applicable. MDBK was purchased from JCRB cell bank, National Institute of Biomedical Innovation, Health and Nutrition. T. b. brucei GUTat3.1, T. b. gambiense IL1922, T. b. rhodesiense IL1501, T. congolense IL3000, and T. evansi Tansui were provided by Dr. Hirumi. T. equiperdum IVM-t1 was established by Dr. Suganuma.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11030_2023_10704_MOESM1_ESM.docx
Supplementary file1—Names and structures of test compounds, the predicted physiochemical and pharmacokinetic properties of the test compounds (DOCX 1054 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janse van Rensburg, H.D., Suganuma, K. & N’Da, D.D. In vitro trypanocidal activities and structure–activity relationships of ciprofloxacin analogs. Mol Divers (2023). https://doi.org/10.1007/s11030-023-10704-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10704-9