Abstract
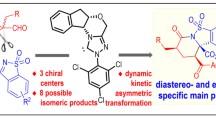
Cyrene, a green bioderived solvent from waste cellulose, was applied to the synthesis of novel α-acyloxyamide derivatives through a Passerini-3CR with carboxylic acids and isocyanides with good yields and diastereoselectivities under mild conditions. Cyrene showed exceptionally high reactivity and the degree of diastereoselection was dependent mostly on the isocyanide. DFT calculations as well as the experimental findings indicated that both kinetic and thermodynamic effects might explain the results.
Graphical abstract














Similar content being viewed by others
References
Hulme C, Dietrich J (2009) Emerging molecular diversity from the intra-molecular Ugi reaction: iterative efficiency in medicinal chemistry. Mol Divers 13:195–207. https://doi.org/10.1007/s11030-009-9111-6
Banfi L, Basso A, Lambruschini C, Moni L, Riva R (2021) The 100 facets of the Passerini reaction. Chem Sci 12:15445–15472. https://doi.org/10.1039/d1sc03810a
Lambruschini C, Moni L, Banfi L (2020) Diastereoselectivity in Passerini reactions of chiral aldehydes and in Ugi reactions of chiral cyclic imines. Eur J Org Chem 2020:3766–3778. https://doi.org/10.1002/ejoc.202000016
Vlahoviček-Kahlina K, Vazdar M, Jakas A et al (2018) Synthesis of glycomimetics by diastereoselective Passerini reaction. J Org Chem 83:13146–13156. https://doi.org/10.1021/acs.joc.8b01874
Forconesi GV, Banfi L, Basso A et al (2020) Synthesis of polyoxygenated heterocycles by diastereoselective functionalization of a bio-based chiral aldehyde exploiting the Passerini reaction. Molecules 25:3227. https://doi.org/10.3390/molecules25143227
Moni L, Banfi L, Basso A et al (2016) Diastereoselective Passerini reaction of biobased chiral aldehydes: divergent synthesis of various polyfunctionalized heterocycles. Org Lett 18:1638–1641. https://doi.org/10.1021/acs.orglett.6b00487
Moni L, Banfi L, Cartagenova D et al (2020) Zinc(II)-mediated diastereoselective Passerini reactions of biocatalytically desymmetrised renewable inputs. Org Chem Front 7:380–398. https://doi.org/10.1039/c9qo00773c
Li C-J, Anastas PT (2012) Green chemistry: present and future. Chem Soc Rev 41:1413–1414. https://doi.org/10.1039/c1cs90064a
Brun N, Hesemann P, Esposito D (2017) Expanding the biomass derived chemical space. Chem Sci 8:4724–4738. https://doi.org/10.1039/C7SC00936D
Cao F, Schwartz TJ, McClelland DJ et al (2015) Dehydration of cellulose to levoglucosenone using polar aprotic solvents. Energy Environ Sci 8:1808–1815. https://doi.org/10.1039/C5EE00353A
Koseki K, Ebata T, Kawakami H et al (1992) US Patent 5,112,994
Court GR, Lawrence CH, Raverty WD, Duncan AJ (2012) US Patent 2012/0111714A1
Kong D, Dolzhenko AV (2022) Cyrene: A bio-based sustainable solvent for organic synthesis. Sust Chem Pharm 25:100591. https://doi.org/10.1016/j.scp.2021.100591
Camp JE (2018) Bio-available solvent cyrene: synthesis, derivatization, and applications. Chemsuschem 11:3048–3055. https://doi.org/10.1002/cssc.201801420
Stini NA, Gkizis PL, Kokotos CG (2022) Cyrene: a bio-based novel and sustainable solvent for organic synthesis. Green Chem 24:6435–6449. https://doi.org/10.1039/D2GC02332F
Circa Group (2017) Safety data sheet: CyreneTM. https://www.sigmaaldrich.com/BR/en/sds/SIAL/807796
Sherwood J, de Bruyn M, Constantinou A et al (2014) Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem Commun 50:9650–9652. https://doi.org/10.1039/C4CC04133J
Prat D, Wells A, Hayler J et al (2016) CHEM21 selection guide of classical- and less classical-solvents. Green Chem 18:288–296. https://doi.org/10.1039/C5GC01008J
Sangon S, Supanchaiyamat N, Sherwood J et al (2020) Direct comparison of safer or sustainable alternative dipolar aprotic solvents for use in carbon–carbon bond formation. React Chem Eng 5:1798–1804. https://doi.org/10.1039/D0RE00174K
Wilson KL, Kennedy AR, Murray J et al (2016) Scope and limitations of a DMF bio-alternative within Sonogashira cross-coupling and Cacchi-type annulation. Beilstein J Org Chem 12:2005–2011. https://doi.org/10.3762/bjoc.12.187
Wilson K, Murray J, Jamieson C, Watson A (2018) Cyrene as a bio-based solvent for the Suzuki–Miyaura cross-coupling. Synlett 29:650–654. https://doi.org/10.1055/s-0036-1589143
Mistry L, Mapesa K, Bousfield TW, Camp JE (2017) Synthesis of ureas in the bio-alternative solvent Cyrene. Green Chem 19:2123–2128. https://doi.org/10.1039/C7GC00908A
Bousfield TW, Pearce KPR, Nyamini SB et al (2019) Synthesis of amides from acid chlorides and amines in the bio-based solvent Cyrene™. Green Chem 21:3675–3681. https://doi.org/10.1039/C9GC01180C
Wilson KL, Murray J, Jamieson C, Watson AJB (2018) Cyrene as a bio-based solvent for HATU mediated amide coupling. Org Biomol Chem 16:2851–2854. https://doi.org/10.1039/C8OB00653A
Nickisch R, Conen P, Gabrielsen SM, Meier MAR (2021) A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv 11:3134–3142. https://doi.org/10.1039/D0RA10436A
Zhang J, White GB, Ryan MD et al (2016) Dihydrolevoglucosenone (Cyrene) as a green alternative to N,N-dimethylformamide (DMF) in MOF synthesis. ACS Sust Chem Eng 4:7186–7192. https://doi.org/10.1021/acssuschemeng.6b02115
Bonneau G, Peru AAM, Flourat AL, Allais F (2018) Organic solvent- and catalyst-free Baeyer-Villiger oxidation of levoglucosenone and dihydrolevoglucosenone (Cyrene®): a sustainable route to (S)-γ-hydroxymethyl-α,β-butenolide and (S)-γ-hydroxymethyl-γ-butyrolactone. Green Chem 20:2455–2458. https://doi.org/10.1039/C8GC00553B
Alhifthi A, Harris BL, Goerigk L et al (2017) Structure–reactivity correlations of the abnormal Beckmann reaction of dihydrolevoglucosenone oxime. Org Biomol Chem 15:10105–10115. https://doi.org/10.1039/C7OB02499A
Hughes L, McElroy CR, Whitwood AC, Hunt AJ (2018) Development of pharmaceutically relevant bio-based intermediates though aldol condensation and Claisen–Schmidt reactions of dihydrolevoglucosenone (Cyrene®). Green Chem 20:4423–4427. https://doi.org/10.1039/C8GC01227J
Hohol RE, Arcure H, Witczak ZJ et al (2018) One-pot synthesis of carbohydrate exo-cyclic enones and hemiketals with 6,8-dioxabicyclo-[3.2.1]octane moieties. Serendipitous formation of a spironolactone when 2-pyridinecarboxaldehyde is used as the reactant Part II. Tetrahedron 74:7303–7309. https://doi.org/10.1016/j.tet.2018.10.049
Ledingham ET, Stockton KP, Greatrex BW (2017) Efficient synthesis of an indinavir precursor from biomass-derived (−)-levoglucosenone. Aust J Chem 70:1146–1150. https://doi.org/10.1071/CH17227
Tsypysheva LP, Valeev FA, Spirikhin LV, Tolstikov GA (2000) Stereochemieal differentiation in the reactions of organometallic reagents with levoglucosenone and some of its dihydro derivatives. Russ Chem BuIl Int Ed 49:1237–1240. https://doi.org/10.1007/BF02495766
Jung ME, Kiankarimi M (1998) Synthesis of methylene-expanded 2′,3″-dideoxyribonucleosides. J Org Chem 63:8133–8144. https://doi.org/10.1021/jo980436l
Andrade CKZ, Takada SCS, Suarez PAZ, Alves MB (2006) Revisiting the Passerini reaction under eco-friendly reaction conditions. Synlett 2006:1535–1539. https://doi.org/10.1055/s-2006-941606
Barreto AFS, Vercillo OE, Andrade CKZ (2011) Microwave-assisted Passerini reactions under solvent-free conditions. J Braz Chem Soc 22:462–467. https://doi.org/10.1590/S0103-50532011000300008
Martinho LA, Rosalba TPF, Andrade CKZ (2022) Passerini reaction to access α-hydroxy amides by facile decarbonylation/decarboxylation of oxalic acid. Eur J Org Chem 2022:e202201199. https://doi.org/10.1002/ejoc.202201199
Ramozzi R, Morokuma K (2015) Revisiting the Passerini reaction mechanism: existence of the nitrilium, organocatalysis of its formation, and solvent effect. J Org Chem 80:5652–5657. https://doi.org/10.1021/acs.joc.5b00594
Frisch MJ, Trucks GW, Schlegel H et al (2016) Gaussian 16, Rev. C.01. Gaussian Inc., Wallingford, 2016.
Bürgi HB, Dunitz JD, Lehn JM, Wipff G (1974) Stereochemistry of reaction paths at carbonyl centres. Tetrahedron 30:1563–1572. https://doi.org/10.1016/S0040-4020(01)90678-7
Acknowledgements
The authors thank Universidade de Brasília (Edital DPG 001/2022), FAPDF (Edital 03/2021) and CAPES for financial support, and Sigma-Aldrich Brazil for a sample of Cyrene.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11030_2023_10618_MOESM1_ESM.docx
Supplementary file1 Typical experimental procedures, NMR and mass spectra of all compounds (PDF), HPLC data for the mixtures of isomers, computational details and crystallographic data for 3a (CIF) (DOCX 150468 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martinho, L.A., Rosalba, T.P.F., Sousa, G.G. et al. Cyrene: a very reactive bio-based chiral ketone in diastereoselective Passerini reactions. Mol Divers 28, 111–123 (2024). https://doi.org/10.1007/s11030-023-10618-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-023-10618-6