Abstract
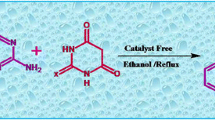
A one pot three component reaction of acenaphthoquinone, barbituric acid/thiobarbituric acid/N,N-dimethyl barbituric acid and arylamines in ethanol for the synthesis of acenaphthoindolopyrimidine derivatives is reported. The reactions take place without a catalyst and gentle conditions. This method is facile and has some benefits such as, readily available starting materials, green solvent, catalyst-free, no column chromatographic purification and good to high yields.
Graphical abstract







Similar content being viewed by others
References
Saher L, Makhloufi-Chebli M, Dermeche L, Dermeche S, Boutemeur-Khedis B, Rabia CH, Hamdi M, Silva A (2018) 10-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-3-methyl 1H,10H-pyrano[4,3-b]chromen-1-ones from a pseudo-multicomponent reaction and evaluation of their antioxidant activity. Tetrahedron 74:872–879. https://doi.org/10.1016/j.tet.2018.01.009
Dabiri M, Tisseh ZN, Bahramnejad M, Bazgir A (2011) Sonochemical multi-component synthesis of spirooxindoles. Ultrason Sonochem 18:1153–1159. https://doi.org/10.1016/j.ultsonch.2010.12.004
Hasaninejad A, Zare A, Shekouhy M (2011) Highly efficient synthesis of triazolo[1,2-a]indazole-triones and novel spiro triazolo[1,2-a]indazole-tetraones under solvent-free conditions. Tetrahedron 67:390–400. https://doi.org/10.1016/j.tet.2010.11.029
Maleki B, Esmailian G, Tayebee R (2015) One-pot synthesis of polysubstituted imidazoles catalyzed by an ionic liquid. Org Prep Proc Int 47:461–472. https://doi.org/10.1080/00304948.2015.1088757
Beyrati M, Forutan M, Hasaninejad A, Rakovský E, Babaei S, Maryamabadi A, Mohebbi GH (2017) One-pot, four-component synthesis of spiroindoloquinazoline derivatives as phospholipase inhibitors. Tetrahedron 73:5144–5152. https://doi.org/10.1016/j.tet.2017.07.005
Zhu J, Bienayme H (2005) Multicomponent reactions. Wiley-VCH, Weinheim
Harichandran G, Amalraj S, Shanmugam P (2018) Amberlite IRA-400 Cl resin catalyzed synthesis of secondary amines and transformation into N-((1Hindol-3-yl) (heteroaryl) methyl)-N-heteroaryl benzenamines and bis-indoles via multicomponent reaction. J Saudi Chem Soc 22:208–217
Zhu G, Huang D, Cao W, Song H, You A (2018) An ab initio study and the corresponded instructing improvement of the multicomponent reaction consisted of acetone, aniline and 4-hydrocoumarine. Comput Theor Chem 1145:22–27. https://doi.org/10.1016/j.comptc.2018.10.010
Wiemann J, Heller L, Csuk R (2018) An access to a library of novel triterpene derivatives with a promising pharmacological potential by Ugi and Passerini multicomponent reactions. Eur J Med Chem 150:176–194. https://doi.org/10.1016/j.ejmech.2018.02.060
Bayat M, Hosseini F, Notash B (2018) Stereoselective synthesis of indenone-fused heterocyclic compounds via a one-pot four-component reaction. Tetrahedron Lett 73:1196–1204. https://doi.org/10.1016/j.tet.2017.01.024
Lazareno S, Popham A, Birdsall NJ (2002) Analogs of win 62,577 define a second allosteric site on muscarinic receptors. Mol Pharmacol 62:1492–1505. https://doi.org/10.1124/mol.62.6.1492
Shaaban MR, Saleh TS, Mayhoub AS, Farag AM (2011) Single step synthesis of new fused pyrimidine derivatives and their evaluation as potent Aurora-A kinase inhibitors. Eur J Med Chem 46:3690–3695. https://doi.org/10.1016/j.ejmech.2011.05.033
Palkar M, Noolvi M, Sankangoud R, Maddi V, Gadad A, Nargund LV (2010) Synthesis and antibacterial activity of a novel series of 2,3-diaryl-substituted-imidazo[2,1-b] benzothiazole derivatives. Arch Pharm 343:353–359. https://doi.org/10.1002/ardp.200900260
Algul O, Meric A, Polat S, Didem YN, Serin MS (2009) Comparative studies on conventional and microwave-assisted synthesis of a series of 2,4-di and 2,3,4-trisubstituted benzimidazo[1,2-a]pyrimidines and their antimicrobial activities. Cent Eur J Chem 7:337–342. https://doi.org/10.2478/s11532-009-0023-1
Murineddu G, Loriga G, Gavini E, Peanna AT, Mule AC, Pinna GA (2001) Synthesis and analgesic-antiinflammatory activities of novel acylarylhydrazones with a 5-phenyl-4-R-3-pyrrolyl-acyl moiety. Arch Pharm 334:393–398. https://doi.org/10.1002/1521-4184(200112)334
Lehuede J, Fauconneau B, Barrier L, Ourakow M, Piriou A, Vierfond JM (1999) Synthesis and antioxidant activity of new tetraarylpyrroles. Eur J Med Chem 34:991–996. https://doi.org/10.1016/s0223-5234(99)00111-7
Khan I, Zaib S, Ibrar A (2020) New frontiers in the transition-metal-free synthesis of heterocycles from alkynoates: an overview and current status. Org Chem Front 7:3734–3791. https://doi.org/10.1039/D0QO00698J
Zhang YC, Jiang F, Shi F (2020) Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: strategies, reactions, and outreach. Acc Chem Res 53(2):425–446. https://doi.org/10.1021/acs.accounts.9b00549
Sheng FT, Wang JY, Tan W, Zhang YC, Shi F (2020) Progresses in organocatalytic asymmetric dearomatization reactions of indole derivatives. Org Chem Front 7:3967–3998. https://doi.org/10.1039/D0QO01124J
Wang Y, Cobo AA, Franz AK (2021) Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org Chem Front 8:4315–4348. https://doi.org/10.1039/D1QO00220A
Michaudel Q, Thevenet D, Baran PS (2012) Intermolecular Rittertype C-H amination of unactivated sp3 carbons. J Am Chem Soc 134:2547–2550. https://doi.org/10.1021/ja212020b
Iglesias A, Alvarez R, Lea AR, Muniz K (2012) Palladium-catalyzed intermolecular C(sp3)–H amidation. Angew Chem Int Ed 51:2225–2228. https://doi.org/10.1002/anie.201108351
Chen WL, Cai YF, Fu X, Liu XH, Liu LL, Feng XM (2011) Enantioselective one-pot synthesis of 2-amino-4-(indol-3-yl)-4Hchromenes. Org Lett 13:4910–4913. https://doi.org/10.1021/ol2019949
Shi F, Xing GJ, Zhu RY, Tan W, Tu SJ (2013) A catalytic asymmetric isatin-involved Povarov reaction: diastereo- and enantioselective construction of spiro[indolin-3,2-quinoline] scaffold. Org Lett 15:128–131. https://doi.org/10.1021/ol303154k
Bayat M, Amiri Z (2018) Catalyst-free synthesis of tetrahydroacenaphtho[1,2-b]indolone derivatives via one-pot four-component reaction. J Heterocycl Chem 55:1346–1351. https://doi.org/10.1002/jhet.3167
Yavari I, Baoosi L, Halvagar MR (2017) A convenient synthesis of fused tetrahydroazocines from acenaphthylene-1,2-dione, proline, and acetylenic esters. Mol Divers 21:257–263. https://doi.org/10.1055/s-0036-1591855
Chen XB, Luo TB, Gou GZ, Wang J, Liu W, Lin J (2015) selective synthesis of acenaphtho[1,2-b]indole derivatives via tandem regioselective aza-ene addition/N cyclization/sN1 type reaction. Asian J Org Chem 4:921–928. https://doi.org/10.1002/ajoc.201500159
Kumar RS, Osman H, Perumal S, Menéndez C, Afshar Ali M, Ismail R, Choon TS (2011) A facile three-component [3+2] cycloaddition/annulation domino protocol for the regio- and diastereoselective synthesis of novel penta- and hexacyclic cage systems, involving the generation of two heterocyclic rings and five contiguous stereocenters. Tetrahedron 67:3132–3139. https://doi.org/10.1016/j.tet.2011.02.058
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kakavand, N., Bayat, M. & Bayat, Y. Catalyst-free synthesis of acenaphthoindolopyrimidine derivatives. Mol Divers 27, 1785–1793 (2023). https://doi.org/10.1007/s11030-022-10531-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10531-4