Abstract
Herein, an effectual, quick and novel method is described for the synthesis of new triazolo[1,5-a]pyrimidine, triazolo[5,1-b][1,3] thiazine and pyrazolo[1,5-a]pyrimidine derivatives. This series of fused six-member rings to triazole and pyrazole was prepared via the catalyst-free reaction of dialkyl acetylenedicarboxylates and 3-substituted 1H-1,2,4-triazole or 3-amino-1H-pyrazole-4-carbonitrile. The structures of the prepared products were deduced from their Fourier-transform infrared, elemental analysis and proton and carbon-13 nuclear magnetic resonance spectral data.
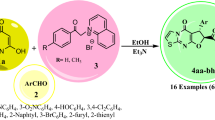
Graphical abstract
A novel and green method is described for the synthesis of new triazolo[1,5-a]pyrimidine, triazolo[5,1-b][1,3] thiazine and pyrazolo[1,5-a]pyrimidine derivatives.




Similar content being viewed by others
References
Jin Z (2013) Muscarine, imidaozle, oxazole and thiazole alkaloids. Nat Prod Rep 30:869–915
Asif M (2014) A mini review on antimalarial activities of biologically active substituted triazole derivatives. Int J Adv Res Chem Sci 1:22–28
Galloway WRJD, Isidro-Llobet A, Spring DR (2010) Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat Commun 1:80–93
Sagar R, Moon-Ju K, Park SB (2008) Ultrasound-promoted one-Pot, three component synthesis of novel 5-amino-2-(4-chlorophenyl)-7-substituted phenyl-8,8a-dihydro-7H-[1,3,4]thiadiazolo[3,2-A]pyrimidine-6-carbonitrile derivatives. Tetrahedron Lett 49:5080–5083
Abdel-Rahman AH, Keshk EM, Hanna MA et al (2004) Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents. Bioorg Med Chem 12:2483–2488
Rai US, Isloor AM, Shetty P et al (2010) Novel chromeno [2,3-b]-pyrimidine derivatives as potential anti-microbial agents. Eur J Med Chem 45:2695–2699
Saito T, Obitsu T, Minamoto C et al (2011) Pyrazolo[1,5-a]pyrimidines, triazolo[1,5-a]pyrimidines and their tricyclic derivatives as corticotropin-releasing factor 1 (CRF1) receptor antagonists. Bioorg Med Chem 19:5955–5966
Yu W, Goddard C, Clearfield E et al (2011) Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg). Secret J Med Chem 54:5660–5670
Shaabani A, Seyyedhamzeh M, Ganji N, Hamidzad Sangachin M, Armaghan M (2015) One-pot four-component synthesis of highly substituted [1,2,4]triazolo[1,5-a]pyrimidines. Mol Divers 19(4):709–715
Salameh S, Abul-Haj M, Quirós M, Salas JM (2005) 1,2,4-triazolo[4,3-a]pyrimidines: a new kind of ligands. Structure of the silver(I) dimer with the 7-oxo derivative. Inorg Chim Acta 358:824–827
Boutaleb-Charki S, Marín C, Maldonado CR, Rosales MJ, Urbano J, Guitierrez-Sánchez R (2009) Copper(II) complexes of [1,2,4]triazolo [1,5-a]pyrimidine derivatives as potential anti-parasitic agents. Drug Metab Lett 3:35–44
Ruisi G, Canfora L, Bruno G, Rotondo A, Mastropietro TF, Debbia EA (2010) Triorganotin(IV) derivatives of 7-amino-2-(methylthio)[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylic acid. Synthesis, spectroscopic characterization, in vitro antimicrobial activity and X-ray crystallography. J Organomet Chem 695:546–551
El-Gendy MM, Shaaban M, Shaaban KA, El-Bondkly AM, Laatsch H (2008) Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J Antibiot 61:149–157
Fizer M, Slivka I (2016) Synthesis of [1,2,4]triazolo[1,5-a]pyrimidine (microreview). Chem Heterocycl Compd 52(3):155–157
Saito T, Obitsu T, Minamoto C, Sugiura T, Matsumura N, Ueno S, Kishi A (2011) Pyrazolo[1,5-a]pyrimidines, triazolo[1,5-a]pyrimidines and their tricyclic derivatives as corticotropin-releasing factor 1 (CRF1) receptor antagonists. Bioorg Med Chem 19:5955–5966
Velders AH, Bergamo A, Alessio E, Zangrando E, Haasnoot JG, Casarsa C, Cocchietto M (2004) Synthesis and chemical–pharmacological characterization of the antimetastatic NAMI-A-type Ru(III) complexes (Hdmtp)[trans-RuCl4(dmso-S)(dmtp)], (Na)[trans-RuCl4(dmso-S)(dmtp)], and [mer-RuCl3(H2O)(dmso-S)(dmtp)] (dmtp = 5,7-Dimethyl[1,2,4]triazolo[1,5-a]pyrimidine). J Med Chem 47:1110–1121
Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk P, Krieter Z, Chen SJ, Russek TT (2006) The anxioselective agent 7-(2-Chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-Gated currents at α1 subunit-containing GABAA receptors. J Pharmacol Exp Ther 319:1244–1252
Raj KKV, Narayan BV, Ashalatha N, Suchita K (2006) New thiazoles containing pyrazolopyrimidine moiety as possible analgesic agents. J Pharmacol Toxicol 1:559–565
Almansa C, Merlos M, Rafanell JGA, Arriba F, Cavalcanti FL, Gomez LA, Miralles A (2001) Synthesis and SAR of a new series of COX-2-Selective inhibitors: pyrazolo[1,5-a]pyrimidines. J Med Chem 44:350–361
Kim DC, Lee YR, Yang B, Shin KJ, Kim DJ, Chung BY, Yoo KH (2003) Synthesis and biological evaluations of pyrazolo[3,4-d]pyrimidines as cyclin-dependent kinase 2 inhibitors. Eur J Med Chem 38:525–532
Xu Y, Brenning BG, Kultgen SG, Foulks JM, Clifford A, Lai Sh (2015) Synthesis and biological evaluation of pyrazolo[1,5-a]pyrimidine compounds as potent and selective pim-1 inhibitors. ACS Med Chem Lett 6(1):63–67
Cervantes-Gomez F, Chen LS, Orlowski RZ, Gandhi V (2013) Biological effects of the Pim kinase inhibitor, SGI-1776, in multiple myeloma. Clin Lymphoma Myeloma Leuk 13:317–329
Wang H, Lee M, Peng Zh, Blázquez B, Lastochkin E, Kumarasiri M, Bouley R (2015) Synthesis and evaluation of 1,2,4-Triazolo[1,5-a]pyrimidines as antibacterial agents against enterococcus faecium. J Med Chem 58:4194–4203
Gavrin LK, Lee A, Provencher BA (2007) Synthesis of pyrazolo[1,5-α]pyrimidinone regioisomers. J Org Chem 72:1043–1046
Dalinger IL, Vatsadse IA, Shevelev SA (2005) Liquid-phase synthesis of combinatorial libraries based on 7-trifluoromethyl-substituted pyrazolo[1,5-a]pyrimidine scaffold. J Comb Chem 7:236–245
Kryl’skii DV, Chuvashlev AS, Arzamastsev AP, Slivkin AI (2009) Synthesis of new pyrazolo[1,5-a]pyrimidines. Pharm Chem J 43:294–296
Verma GK, Raghuvanshi K, Verma RK, Dwivedi P, Singh MS (2011) An efficient one-pot solvent-free synthesis and photophysical properties of 9-aryl/alkyl-octahydroxanthene-1,8-diones. Tetrahedron 67(20):3698–3704
Duarte RCC, Ribeiro MTC, Machado AASC (2017) Reaction scale and green chemistry: Microscale or macroscale, which is greener? J Chem Educ 94(9):1255–1264
Karami B, Farahi M, Banaki Z (2015) A new protocol for catalyst-free regioselective synthesis of 5,9-dihydropyrimido[5,4-e][1,2,4]triazolo[1,5-a]pyrimidine-6,8(4H,7H)-diones. Synlett 26(06):741–744
Karami B, Farahi M, Banaki Z (2015) A novel one-pot method for highly regioselective synthesis of triazoloapyrimidinedicarboxylates using silica sodium carbonate. Synlett 26(13):1804–1807
Karami B, Farahi M, Akrami S, Elhamifar D (2018) Tungstic acid-functionalized MCM-41 as a novel mesoporous solid acid catalyst for the one-pot synthesis of new pyrrolo[2,1-a]isoquinolines. New J Chem 42:12811–12816
Akrami S, Karami B, Farahi M (2017) Preparation and characterization of novel phthalhydrazide-functionalized MCM-41 and its application in the one-pot synthesis of coumarin-fused triazolopyrimidines. RSC Adv 7:34315–34320
Eskandari Kh, Karami B, Farahi M, Mouzari V (2016) Silica sodium carbonate catalyzed in water synthesis of novel benzylbarbiturocoumarin derivatives. Tetrahedron Lett 57:487–491
Farahi M, Karami B, Mohamadi Tanuraghaj H (2015) Efficient synthesis of a new class of sulfonamide-substituted coumarins. Tetrahedron Lett 56:1833–1836
Karami B, Farahi M, Farmani N, Mohamadi Tanuraghaj H (2016) Novel synthesis of coumarin-containing secondary benzamide derivatives using tungstate sulfuric acid. New J Chem 40:1715–1719
Farahi M, Tamaddon F, Karami B, Pasdar S (2015) Highly efficient syntheses of α-amino ketones and pentasubstituted pyrroles using reusable heterogeneous catalysts. Tetrahedron Lett 56:1887–1890
Neochoritis CG, Zarganes-Tzitzikas T, Stephanidou-Stephanatou J (2014) Dimethyl acetylenedicarboxylate: a versatile tool in organic synthesis. Synthesis 46:537–585
Acknowledgements
The authors gratefully acknowledge partial support of this work by Yasouj University, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akrami, S., Karami, B. & Farahi, M. A novel protocol for catalyst-free synthesis of fused six-member rings to triazole and pyrazole. Mol Divers 24, 225–231 (2020). https://doi.org/10.1007/s11030-019-09944-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09944-5