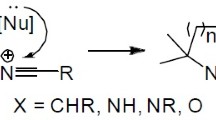
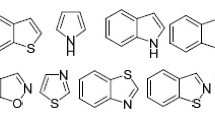
Abstract
The favorability of ring closure reactions as per Baldwin rules has gained immense importance recently. This is evident from the current literature such as research articles, reviews, and books that have been published in this area. This review covers the recent applications of 5-endo-dig cyclization in organic synthesis focusing in the last two decades. A variety of 5-membered heterocycles as well as carbocycles could be synthesized via 5-endo-dig cyclization reactions. The important applications of 5-endo-dig cyclization in organic synthesis covering different aspects have been summarized in this review.
Graphical abstract















































Similar content being viewed by others
References
Baldwin JE (1976) Rules for ring closure. J Chem Soc Chem Comm. https://doi.org/10.1039/C39760000734
Gilmore K, Alabugin IV (2011) Cyclizations of alkynes: revisiting Baldwin’s rules for ring closure. Chem Rev 111:6513–6556. https://doi.org/10.1021/cr200164y
Gilmore K, Mohamed RK, Alabugin IV (2016) The Baldwin rules: revised and extended. WIREs Comput Mol Sci 6:487–514. https://doi.org/10.1002/wcms.1261
Alabugin IV, Timokhin VI, Abrams JN, Manoharan M, Abrams R, Ghiviriga I (2008) In search of efficient 5-endo-dig cyclization of a carbon-centered radical: 40 Years from a prediction to another success for the Baldwin rules. J Am Chem Soc 130:10984–10995. https://doi.org/10.1021/ja801478n
Alabugin IV, Manoharan M (2005) 5-Endo-dig radical cyclizations: “The poor cousins” of the radical cyclizations family. J Am Chem Soc 127:9534–9545. https://doi.org/10.1021/ja050976h
Ojima I, Tzamarioudaki M, Li Z, Donovan RJ (1996) Transition metal-catalyzed carbocyclizations in organic synthesis. Chem Rev 96:635–662. https://doi.org/10.1021/cr950065y
Rode ND, Aschi M, Chiarini M, Vecchio LD, Marinelli F, Arcadi A (2018) Reaction of β-(2-aminophenyl)-α,β-ynones with tosyl isocyanate: experimental and computational investigations. Adv Synth Catal 360:3672–3676. https://doi.org/10.1002/adsc.201800733
Rodriguez AL, Koradin C, Dohle W, Knochel P (2000) Versatile indole synthesis by a 5-endo-dig cyclization mediated by potassium or cesium bases. Angew Chem Int Ed 39:2488–2490. https://doi.org/10.1002/1521-3773(20000717)39:14%3c2488:AID-ANIE2488%3e3.0.CO;2-E
Takahashi S, Kuroyama Y, Sonogashira K, Hagihara N (1980) A convenient synthesis of ethynylarenes and di-ethynylarenes. Synthesis 1980:627–630. https://doi.org/10.1055/s-1980-29145
Andreev IA, Ratmanova NK, Novoselov AM, Belov DS, Seregina IF, Kurkin AV (2016) Oxidative dearomatization of 4,5,6,7-tetrahydro-1H-indoles obtained by metal- and solvent-free thermal 5-endo-dig cyclization: the route to erythrina and lycorine alkaloids. Chem Eur J 22:7262–7267. https://doi.org/10.1002/chem.201600273
Naoe S, Saito T, Uchiyama M, Oishi S, Fujii N, Ohno H (2015) Direct construction of fused indoles by gold-catalyzed cascade cyclization of conjugated diynes. Org Lett 17:1774–1777. https://doi.org/10.1021/acs.orglett.5b00550
Jalal S, Paul K, Jana U (2016) Iron-catalyzed 1,5-enyne cycloisomerization via 5-endo-dig cyclization for the synthesis of 3-(inden-1-yl)indole derivatives. Org Lett 18:6512–6515. https://doi.org/10.1021/acs.orglett.6b03544
Sharma S, Pathare RS, Sukanya Maurya AK, Goswami B, Agnihotri VK, Sawant DM, Pardasani RT (2017) Microwave assisted metal-/oxidant-free cascade electrophilic sulfenylation/5-endo-dig cyclization of 2-alkynylanilines to generate diversified 3-sulfenylindoles. Tetrahedron Lett 58:3823–3826. https://doi.org/10.1016/j.tetlet.2017.08.046
Palimkar SS, Kumar PH, Lahoti RJ, Srinivasan KV (2006) Ligand-, copper-, and amine-free one-pot synthesis of 2-substituted indoles via Sonogashira coupling 5-endo-dig cyclization. Tetrahedron 62:5109–5115. https://doi.org/10.1016/j.tet.2006.03.035
Palimkar SS, More VS, Kumar PH, Srinivasan KV (2007) Synthesis of an indole containing KDR kinase inhibitor by tandem Sonogashira coupling-5-endo-dig-cyclization as a key step. Tetrahedron 63:12786–12790. https://doi.org/10.1016/j.tet.2007.09.075
Kuethe JT, Wong A, Qu C, Smitrovich J, Davies IW, Hughes DL (2005) Synthesis of 5-substituted-1H-indol-2-yl-1H-quinolin-2-ones: a novel class of KDR kinase inhibitors. J Org Chem 70:2555–2567. https://doi.org/10.1021/jo0480545
Cai J, Wu B, Rong G, Zhang C, Qiu L, Xu X (2018) Gold-catalyzed bicyclization of diaryl alkynes: synthesis of polycyclic fused indole and spirooxindole derivatives. Org Lett 20:2733–2736. https://doi.org/10.1021/acs.orglett.8b00939
Jaimes MCB, Weingand V, Rominger F, Hashmi ASK (2013) From ynamides to highly substituted benzo[b]furans: gold(I)-catalyzed 5-endo-dig-cyclization/rearrangement of alkylic oxonium intermediates. Chem Eur J 19:12504–12511. https://doi.org/10.1002/chem.201301595
Saha D, Dey R, Ranu BC (2010) A simple and efficient one-pot synthesis of substituted benzo[b]furans by Sonogashira coupling-5-endo-dig cyclization catalyzed by palladium nanoparticles in water under ligand- and copper-free aerobic conditions. Eur J Org Chem 2010:6067–6071. https://doi.org/10.1002/ejoc.201000980
Mantovani AC, Hernandez JG, Bolm C (2018) Synthesis of 3-iodobenzofurans by electrophilic cyclization under solventless conditions in a ball mill. Eur J Org Chem 2018:2458–2461. https://doi.org/10.1002/ejoc.201800027
Kim I, Kim K, Choi J (2009) A direct approach to 5-hydroxybenzofurans via a platinum-catalyzed domino rearrangement/5-endo-dig cyclization reaction of quinols. J Org Chem 74:8492–8495. https://doi.org/10.1021/jo901937u
Palimkar SS, More VS, Srinivasan KV (2008) Ultrasound promoted copper-, ligand- and amine-free synthesis of benzo[b]furans/nitro benzo[b]furans via Sonogashira coupling-5-endo-dig-cyclization. Ultrason Sonochem 15:853–862. https://doi.org/10.1016/j.ultsonch.2007.10.006
Majumdar KC, Chattopadhyay B, Samanta S (2009) A short route to the synthesis of pyrrolocoumarin and pyrroloquinolone derivatives by sonogashira cross-coupling and gold-catalyzed cycloisomerization of acetylenic amines. Synthesis 2:311–317. https://doi.org/10.1055/s-0028-1083295
Majumdar KC, De N, Sinha B, Roy B (2012) Synthesis of 3-iodopyrrolocoumarins via iodine-induced 5-endo-dig electrophilic cyclization. Monatsh Chem 143:1067–1073. https://doi.org/10.1007/s00706-011-0694-0
Staben ST, Kennedy-Smith JJ, Toste FD (2004) Gold(I)-catalyzed 5-endo-dig carbocyclization of acetylenic dicarbonyl compounds. Angew Chem 116:5464–5466. https://doi.org/10.1002/ange.200460844
Suzuki S, Tokunaga E, Reddy DS, Matsumoto T, Shiro M, Shibata N (2012) Enantioselective 5-endo-dig carbocyclization of β-ketoesters with internal alkynes employing a four-component catalyst system. Angew Chem Int Ed 51:4131–4135. https://doi.org/10.1002/anie.201201060
Dolaine R, Gleason JL (2000) Diastereoselective formation of 5-vinylcyclopentenes from 1,6-enynes: cobalt-mediated C–H allylic activation and formal 5-endo-dig cyclization. Org Lett 2:1753–1756. https://doi.org/10.1021/ol005928a
Lee PH, Lee K, Kim S (2001) A novel nucleophilic substitution of in situ generated 3-tert-butyldimethyl-silyloxyalk-2-enylsulfonium salts with allylindium reagents. Org Lett 3:3205–3207. https://doi.org/10.1021/ol016542i
Iwasawa N, Miura T, Kiyota K, Kusama H, Lee K, Lee PH (2002) An efficient method for cyclopentene annulation onto α,β-unsaturated ketones: W(CO)5(L)-catalyzed 5-endo-dig cyclization of 6-siloxy-5-en-1-ynes. Org Lett 4:4463–4466. https://doi.org/10.1021/ol026993i
Fujino D, Yorimitsu H, Osuka A (2012) Synthesis of 1,2-disubstituted cyclopentenes by palladium-catalyzed reaction of homopropargyl-substituted dicarbonyl compounds with organic halides via 5-endo-dig cyclization. Org Lett 14:2914–2917. https://doi.org/10.1021/ol301257m
French JM, Diver ST (2014) Gold(I)-promoted heterocyclization of internal alkynes: a comparative study of direct metallate 5-endo-dig cyclization versus a stepwise cyclization. J Org Chem 79:5569–5585. https://doi.org/10.1021/jo500748e
Majumdar KC, Ganai S, Nandi RK (2011) Regioselective synthesis of pyrimidine-annulated spiro-dihydrofurans by silver-catalyzed 5-endo-dig cyclization. New J Chem 35:1355–1359. https://doi.org/10.1039/c1nj20121b
Sniady A, Wheeler KA, Dembinski R (2005) 5-Endo-dig electrophilic cyclization of 1,4-disubstituted but-3-yn-1-ones: regiocontrolled synthesis of 2,5-disubstituted 3-bromo- and 3-iodofurans. Org Lett 7:1769–1772. https://doi.org/10.1021/ol050372i
Sniady A, Durham A, Morreale MS, Wheeler KA, Dembinski R (2007) Room temperature zinc chloride-catalyzed cycloisomerization of alk-3-yn-1-ones: synthesis of substituted furans. Org Lett 9:1175–1178. https://doi.org/10.1021/ol062539t
Wakabayashi Y, Fukuda Y, Shiragami H, Utimoto K, Nozaki H (1985) Preparation of furans from alkynols utilizing palladium catalyzed intramolecular addition of alcohol to acetylene as a key reaction. Tetrahedron 41:3655–3661. https://doi.org/10.1016/S0040-4020(01)91384-5
El-Taeb GMM, Evans AB, Jones S, Knight DW (2001) Practical alternatives for the synthesis of β-iodofurans by 5-endo-dig cyclisations of 3-alkyne-1,2-diols. Tetrahedron Lett 42:5945–5948. https://doi.org/10.1016/S0040-4039(01)01112-1
Shi T, Guo X, Teng S, Hu W (2015) Pd(II)-catalyzed formal [4 + 1] cycloadditions of diazoacetates and aryl propargyl alcohols to form 2,5-dihydrofurans. Chem Commun 51:15204–15207. https://doi.org/10.1039/C5CC05000F
Oechsle P, Florke U, Egold H, Paradies J (2016) Heteroacene synthesis through C–S cross-coupling/5-endo-dig cyclization. Chem Eur J 22:18559–18563. https://doi.org/10.1002/chem.201603737
Khan ZA, Wirth T (2009) Synthesis of indene derivatives via electrophilic cyclization. Org Lett 11:229–231. https://doi.org/10.1021/ol8024956
Ma B, Wu Z, Huang B, Liu L, Zhang J (2016) Gold-catalysed facile access to indene scaffold via sequential C–H functionalization and 5-endo-dig carbocyclization. Chem Commun 52:9351–9354. https://doi.org/10.1039/C6CC04034A
Smith CR, Bunnelle EM, Rhodes AJ, Sarpong R (2007) Pt-Catalyzed cyclization/1,2-migration for the synthesis of indolizines, pyrrolones, and indolizinones. Org Lett 9:1169–1171. https://doi.org/10.1021/ol0701971
Pandya AN, Fletcher JT, Villa EM, Agrawal DK (2014) Silver-mediated synthesis of indolizines via oxidative C–H functionalization and 5-endo-dig cyclization. Tetrahedron Lett 55:6922–6924. https://doi.org/10.1016/j.tetlet.2014.10.112
Seregin IV, Gevorgyan V (2006) Gold-catalyzed 1,2-migration of silicon, tin, and germanium en route to C-2 substituted fused pyrrole-containing heterocycles. J Am Chem Soc 128:12050–12051. https://doi.org/10.1021/ja063278l
Kim I, Choi J, Won HK, Lee GH (2007) Expeditious synthesis of indolizine derivatives via iodine mediated 5-endo-dig cyclization. Tetrahedron Lett 48:6863–6867. https://doi.org/10.1016/j.tetlet.2007.07.180
Sugita S, Ueda M, Doi N, Takeda N, Miyata O (2016) Gold-catalyzed sequential cyclization/rearrangement reaction of O-allyl hydroxamates: atom economical synthesis of 3-hydroxyisoxazoles. Tetrahedron Lett 57:1786–1789. https://doi.org/10.1016/j.tetlet.2016.03.032
Wang Q, Tsui GC (2018) Copper-mediated domino cyclization/trifluoromethylation of propargylic N-hydroxylamines: synthesis of 4-trifluoromethyl-4-isoxazolines. J Org Chem 83:2971–2979. https://doi.org/10.1021/acs.joc.7b03191
Xiao D, Han L, Sun Q, Chen Q, Gong N, Lv Y, Suzenet F, Guillaumet G, Chenga T, Li R (2012) Copper-mediated synthesis of N-fused heterocycles via Csp–S coupling reaction and 5-endo-dig cyclization sequence. RSC Adv 2:5054–5057. https://doi.org/10.1039/c2ra20254a
Chen Y, Wang D, Petersen JL, Akhmedov NG, Shi X (2010) Synthesis and characterization of organogold complexes containing an acid stable Au–C bond through triazole-yne 5-endo-dig cyclization. Chem Commun 46:6147–6149. https://doi.org/10.1039/c0cc01338b
Ueda H, Yamaguchi M, Kameya H, Sugimoto K, Tokuyama H (2014) Autotandem catalysis: synthesis of pyrroles by gold-catalyzed cascade reaction. Org Lett 16:4948–4951. https://doi.org/10.1021/ol5024695
Bharathiraja G, Sakthivel S, Sengoden M, Punniyamurthy T (2013) A novel tandem sequence to pyrrole syntheses by 5-endo-dig cyclization of 1,3-enynes with amines. Org Lett 15:4996–4999. https://doi.org/10.1021/ol402305b
Queiroz M-JRP, Begouin A, Pereira G, Ferreira PMT (2008) New synthesis of methyl 5-aryl or heteroaryl pyrrole-2-carboxylates by a tandem Sonogashira coupling/5-endo-dig-cyclization from β-iododehydroamino acid methyl esters and terminal alkynes. Tetrahedron 64:10714–10720. https://doi.org/10.1016/j.tet.2008.08.105
Yan Z-Y, Xiao Y, Zhang L (2012) Gold-catalyzed one-step construction of 2,3-dihydro-1H-pyrrolizines with an electron-withdrawing group in the 5-position: a formal synthesis of 7-methoxymitosene. Angew Chem Int Ed 51:8624–8627. https://doi.org/10.1002/anie.201203678
Gorin DJ, Davis NR, Toste FD (2005) Gold(I)-catalyzed intramolecular acetylenic Schmidt reaction. J Am Chem Soc 127:11260–11261. https://doi.org/10.1021/ja053804t
Postoia RA, Roehrs JA, Back DF, Zeni G (2017) Iodine-mediated regioselective 5-endo-dig electrophilic cyclization reaction of selenoenynes: synthesis of selenophene derivatives. Org Chem Front 4:277–282. https://doi.org/10.1039/x0xx00000x
Adler P, Fadel A, Rabasso N (2015) Cerium(IV) ammonium nitrate mediated 5-endo-dig cyclization of α-amino allenylphosphonates to spirodienones. Chem Commun 51:3612–3615. https://doi.org/10.1039/c5cc00281h
Wu W-T, Xu R-Q, Zhang L, You S-L (2016) Construction of spirocarbocycles via gold-catalyzed intramolecular dearomatization of naphthols. Chem Sci 7:3427–3431. https://doi.org/10.1039/c5sc04130a
Reddy BVS, Swain M, Reddy SM, Yadav JS, Sridhar B (2014) Gold-catalyzed 5-endo-dig cyclization of 2-[(2-aminophenyl)-ethynyl]phenylamine with ketones for the synthesis of spiroindolone and indolo[3,2-c]quinolone scaffolds. Eur J Org Chem 2014:3313–3318. https://doi.org/10.1002/ejoc.201402006
Acknowledgements
The second and third authors are grateful to Professor Dr. Thomas Wirth, Cardiff University, UK, for proof reading and valuable suggestions to improve the manuscript. The corresponding author is grateful to Higher Education Commission, Pakistan (Project No. PM-IPFP/HRD/HEC/2011/2063) and Government College University, Faisalabad (GCUF-RSP Project No. 61-CHM-1) for providing the facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sajid, M.A., Khan, Z.A., Shahzad, S.A. et al. 5-Endo-dig cyclizations in organic syntheses. Mol Divers 24, 295–317 (2020). https://doi.org/10.1007/s11030-019-09930-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09930-x