Abstract
Ocean deoxygenation and expansion and intensification of hypoxia in the ocean are a major, growing threat to marine ecosystems. Measures currently used to protect marine biodiversity (e.g., marine protected areas) are ineffective in countering this threat. Here, we highlight the example of the Gulf of St. Lawrence in eastern Canada, where oxygen loss is not only due to eutrophication (which can be mitigated by nutrient controls) but also is a consequence of ocean circulation change and warming. Climate-related loss of oxygen will be an increasingly widespread source of risk to marine biodiversity over this century. Again using the Gulf of St. Lawrence as an example, we show that production of oxygen by the green hydrogen industry can be comparable to the loss rate of dissolved oxygen on large spatial scales, offering new possibilities for mitigation. However, this mitigation approach has rarely been considered for marine environments to date. Given confluence of increasing risk to marine ecosystems from oxygen loss and rapid emergence, worldwide, of industrial sources of pure oxygen, which are likely to be located in coastal regions, we believe this option will be proposed increasingly in coming years, including by the private sector. We argue that it is urgent for ocean scientists, engineers, and policymakers to recognize and address this emerging potential. A coordinated research effort should be established immediately in order to harness the potential of the green hydrogen industry to mitigate major impacts of climate change on marine biodiversity, and avoid any unintended negative consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background: ocean deoxygenation
As human beings, we do not usually worry about our impact on oxygen within the earth system. Together with other air-breathing terrestrial animals (and marine mammals), we are not impacted by the minimal, but measurable rate at which oxygen is decreasing in the atmosphere due to combustion of fossil fuels (~ 19 per meg/year; Keeling and Manning 2013). The situation is different for underwater life, however, because dissolved oxygen levels vary naturally between oceanic regions, and can reach low levels in restricted regions with high productivity and/or restricted circulation, such as oxygen minimum zones (OMZs). The amount of oxygen dissolved in water exerts major control on life within the ocean, on both metabolism of individual organisms and their habitat (Stramma et al. 2012; Diaz and Rosenberg 2008) and frequently determines whether and what type of life can exist.
The risk of hypoxia (where oxygen depletion impacts organisms) due to eutrophication is widely recognized for freshwater ecosystems and is a frequent focus of mitigation efforts. In comparison, threats to oceanic oxygen now include but extend beyond localized impacts of eutrophication and include threats at regional to global scales arising from climate change. However, awareness of the sensitivity of marine ecosystems to changing oxygen levels is limited and has developed largely over the past two or three decades. Examples of practical efforts or concepts to remediate or mitigate oceanic oxygen depletion are rare (some examples are discussed below), and are largely non-existent at larger scales. However, a recent modelling study did discuss re-oxygenation at the very large geographic scale of the open ocean’s oxygen minimum zones (Beghoura et al. 2023).
Where remediation of oceanic oxygen levels has been considered, it has typically focussed on coastal systems and reduction of nutrient inputs to limit eutrophication and development of coastal “dead zones.” Conceptually, this is an extension of approaches used within lakes, ponds, and reservoirs. Aquaculture operators have also attempted to mitigate local impacts of oxygen depletion on fish production within pens during warmer months.
Natural inputs of oxygen to the ocean are restricted to the sunlit, surface layer of the ocean where photosynthesis can outpace respiration and where oxygen can be taken up from the atmosphere. Once transported beneath this surface layer, oxygen is progressively consumed by aerobic respiration. The consequence is that subsurface oxygen varies from levels close to solubility equilibrium with the atmosphere to near-zero levels in the OMZs and coastal dead zones.
Subsurface oxygen is sensitive to both direct and indirect climate-change impacts involving ocean temperature, circulation, and mixing (Stramma et al. 2008). First of all, warmer water has lower oxygen solubility so surface waters in equilibrium with the atmosphere today carry less oxygen into the ocean interior than they used to. Secondly, warming of the upper ocean and, in some locations, decreasing salinity due to ice melt, cause the ocean to become more stratified, which can decrease the effectiveness of deep currents and mixing processes, such as high-latitude wintertime convection, in delivering oxygen to deepwater ecosystems (Atamanchuk et al. 2022, Koelling et al. 2017; Wolf et al. 2018). More subtle feedbacks may also play a role: for example, animal and microbial metabolism speeds up with increasing temperature which can cause the rate of biological utilization of oxygen to increase (e.g., Kullenberg 1970).
The threat from increased nutrient input and expansion of coastal “dead zones” has been recognized for some time, with Diaz and Rosenberg (2008) noting that they were 40 times more numerous in 2007 than in the 1920s. On the other hand, climate-change impacts have been recognized more recently (Stramma and Schmidtko 2021; Breitburg et al. 2018; Brandt et al. 2015). Analyses of historical oxygen data (Schmidtko et al. 2017) show that large volumes of the subsurface ocean have lost oxygen over the past 50 years, for a total loss of 2% of its global inventory. This loss rate is six times larger than predicted for the effect of warming on oxygen solubility alone (Bopp et al. 2013) and is not fully understood (Oschlies et al. 2018).
Recent model-based projections now warn that, at the global scale, oxygen loss from the ocean coupled with warming will impact marine life to the same extent as fishing by the end of this century and could ultimately “culminate in a mass extinction rivaling those in Earth’s past” unless greenhouse gas emissions are curbed (Penn and Deutsch 2022). Ecosystem impacts of ocean hypoxia have also been the subject of a meta-analysis by Sampaio et al. (2021) who found that it drives consistently negative effects on organism survival, abundance, development, metabolism, growth, and reproduction among the mollusks, crustaceans, and fish studied.
In addition to direct, ecophysiological impacts on marine animals, low oxygen levels can act as “tipping points” for microbial cycling of critical nutrient elements (e.g., phosphorus, nitrogen, iron) or for mobilization of contaminants and metals, with largely unknown consequences for marine biodiversity (Scholz et al. 2014; Raiswell & Canfield 2012; Ingall & Jahnke 1994; Sundby et al. 1992). Oxygen-related changes to biogeochemical cycling can also feedback on climate, affecting budgets of greenhouse gases such as N2O (Kalvelage et al. 2013; Bange et al. 2010).
Despite this growing threat, and although ocean deoxygenation is now recognized as a negative consequence of climate change, most current efforts to address and conserve marine biodiversity focus exclusively on mitigation of the direct human impacts on ecosystems such as fishing, habitat destruction, or localized inputs of nutrients and contaminants. Establishment of marine protected areas (MPAs) is a common approach. However, MPAs are likely to be largely ineffectual in preventing development of low oxygen conditions due to climate forcing. At present, with the exception of a very few studies, such as pioneering research and pilot-scale testing of artificial mitigation of hypoxia in a small Swedish fjord (Stigebrandt et al. 2015), relatively little consideration has been given to the protection of marine biodiversity from this threat. Here, we highlight and critically discuss a new, emerging opportunity to counter hypoxia in coastal waters at large regional scales (e.g., > 105 km2), using oxygen produced as a by-product of the emerging green hydrogen industry. We discuss the concept using the example of the Gulf of St. Lawrence, and consider the ethical issues, potential for unintended consequences, and research needs raised by this mitigation option.
2 The example of growing hypoxia in the St. Lawrence Estuary and Gulf
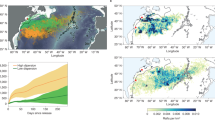
Together, the Lower St. Lawrence Estuary and Gulf of St. Lawrence (LSLE and GoSL; Fig. 1) comprise the largest semi-enclosed estuary in the world, and provide an example of how major regions of the ocean can be threatened by hypoxia due to changes in ocean circulation. Measurements of subsurface waters (> 150 m) made since the 1930s within the Lower St. Lawrence Estuary (LSLE; Fig. 1) reveal a long-term decline of oxygen (Jutras et al. 2023; Genovesi et al. 2011; Thibodeau et al. 2006; Gilbert et al. 2005). This has been caused by eutrophication and, predominantly since 2008, by a circulation-related shift in water mass characteristics at the entry point of Atlantic-derived deep waters in Cabot Strait, between Newfoundland and Nova Scotia (Jutras et al. 2020; Gilbert et al. 2005). The change in water masses outside the GoSL has been connected (Claret et al. 2018) with the climate-related retreat of the southward flowing, oxygen-rich Labrador Current and with slowing of the Atlantic Meridional Overturning Circulation. The decrease in delivery of oxygen resulting from circulation is compounded by associated warming of subsurface waters, which amplifies the oxygen decline due to increased microbial metabolism (Jutras et al. 2020).
a Measured oxygen concentrations and (b) oxygen saturation on the density level of the oxygen minimum (1027.15 to 1027.30 kg/m3) in the LSLE and GoSL. The thin black line delineates the 250-m isobath. The purple star indicates the injection location for the tracer release experiment (see Section 4). The oxygen saturation is calculated from the in situ temperature and salinity. We show data collected between 1980 and 2018. The data originates from the BioChem database from Fisheries and Oceans Canada (DFO 2018; Devine et al. 2014)
The minimum dissolved oxygen concentration within the LSLE declined from ~ 150 µmol/kg in the 1930s to ~ 55–65 µmol/kg in the 2000s, and dropped suddenly to < 35 µmol/kg in 2020 (Jutras et al. 2023). The most recent decline of 20 µmol/kg in only 1 year is stronger than the most rapid deoxygenation observed previously (e.g., 5 µmol/kg/year in the eastern Pacific OMZ, Stramma et al. 2009), emphasizing the strong impact that circulation change can have on oxygen at regional scales.
Oxygen saturation and concentration in the LSLE, near Rimouski (Fig. 1), are now well below levels classified as hypoxic (Fig. 2; typically < 2 mg/L or 62.5 µmol/kg; Diaz and Rosenberg 1995). Waters to the northeast of Anticosti Island have also crossed this threshold in the last 2 years (Figs. 1 and 2) and oxygen concentrations are declining throughout the entire GoSL. Hypoxia in the Gulf of St. Lawrence is expected to reduce the viability, size, and distribution of a number of key species, including cod (Petersen and Gamperl 2010; Chabot and Claireaux 2008; D'Amours 1993), halibut (Dupont-Prinet et al. 2013a), and shrimp (Dupont-Prinet et al. 2013b). The latter two species are important contributors to the regional economy, 30% of which depends on fisheries (DFO 2010).
Dissolved oxygen concentration for transects along the Anticosti Channel (top) and the Laurentian Channel (bottom), using data from 2020 and 2021, from the channel’s head to Cabot Strait (right edge of the plot). Black dots indicate the locations of measurements. The white line delineates the boundary of the hypoxic zone (< 62.5 µmol/kg)
Both the intensity and extent of hypoxia are increasing. The area of the hypoxic zone within the LSLE and GoSL has increased from 1300 km2 in 2003 to more than 9000 km2 in 2021 (Jutras et al. 2023), and now covers 15% of the GoSL deeper than 250 m. Hence, less mobile species are increasingly threatened, as are more mobile species whose habitat is constricted, potentially making them more vulnerable to predation. The indirect consequences on ecosystems of the growing volume of hypoxic water and altered nutrient and contaminant cycling (including sediment–water exchanges) have not yet been assessed.
Based on Gulf-wide surveys conducted over the past 90 years, we estimate that the concentration of oxygen in subsurface waters (> 150 m) in the GoSL has decreased on average by ca. 1 μmol/kg/year (excluding the rapid decline in 2020), with about 0.6 μmol/kg/year of this due to eutrophication and ca. 0.5 μmol/kg/year due to changes of circulation and water mass characteristics. By integrating the eutrophication-driven oxygen loss throughout the Lower St. Lawrence Estuary and the circulation-driven oxygen loss over the entire volume of water within this depth range (> 150 m) in the GoSL (1.2 × 1013 m3), we estimate the total loss of O2 from deep water within the LSLE and GoSL to be about 2 × 105 tonnes of O2 per year.
Our recent observations (Jutras et al. 2023) show that the contribution of colder, oxygen-rich, Labrador Current waters to subsurface waters entering the GoSL has been reduced to near-zero. This suggests that subsurface oxygen will now start to approach a new steady-state distribution with significantly reduced levels. The timescale for this, and the final expected distribution of oxygen once a new steady-state has been reached, has not yet been modelled. However subsurface temperatures may continue to increase in concert with widespread ocean warming (Bates and Johnson 2020; Saba et al. 2016). This may cause oxygen levels to continue to decrease over the long term, but at a slower rate than over the past 50 years, when eutrophication was developing and water mass composition was changing.
3 Can and should anything be done?
Decreasing oxygen levels present a very serious threat to the marine ecosystem and an obvious question is “can anything be done”? A second related question is “should anything be done”? The first is an engineering-related question. If the answer to the first question is “yes” then the second question requires consideration of the consequences of “doing nothing” as well as of costs, impacts, and effectiveness of mitigation. The second question includes consideration of ethics, raised in Section 5, below.
A number of approaches to artificial re-oxygenation of coastal water bodies have been considered, usually theoretically. One of the earliest was a suggestion by Wright et al. (1992) for artificial enhancement of mixing of surface water into deeper waters of the Chesapeake Bay (Eastern USA). Stigebrandt and Andersson (2022) evaluated a variety of sea-based measures for oxygen mitigation within a small Swedish fjord, including reduction of nutrient supply, direct injection of pure oxygen, artificial downwelling and modification of local topography to enhance flows, and turbulent mixing.
Artificial downwelling (pumping of oxygenated surface water into oxygen-deficient subsurface water) and injection of pure oxygen directly into subsurface water are both potentially effective in restoring oxic conditions and promoting ecosystem health in small, semi-enclosed bays and fjords (Stigebrandt and Andersson 2022; Liu et al 2020). However, such schemes involve substantial operating costs as well as up-front costs for infrastructure. The cost of artificial downwelling was estimated to be approximately an order of magnitude lower than that of oxygen injection due to the high cost of pure oxygen (Stigebrandt and Andersson 2022).
Most emphasis to date has been on the potential of mitigation efforts for realizing positive ecosystem benefits, and on costs, although the risk of negative consequences has sometimes also been considered. Risks can arise from changes to nutrient cycling (Koweek et al. 2020; Conley et al. 2009; Gerling et al 2014) and/or effects of warming of subsurface waters and sediments that could even lead to further decrease of oxygen (via accelerated metabolism) rather than mitigation (Conley et al. 2009).
The exact impact of a mitigation technique on dissolved oxygen is hard to predict (Koweek et al. 2020). The effectiveness of engineering approaches, including direct oxygen injection, has been questioned for systems where hypoxia is driven by nutrient inputs, including internal sources and feedbacks as in the Baltic (Stigebrandt and Andersson 2020) and several other major bays and estuaries (e.g., Chesapeake Bay). The LSLE and GoSL, where circulation change rather than nutrient input is increasingly the major driver of oxygen loss, may represent a different situation with different risk considerations and may be particularly relevant, here and elsewhere, for the future in the context of the growing impacts of climate change.
The growing threat of oxygen loss globally, as exemplified by the situation in the LSLE and GoSL, suggests it is time to reconsider the potential for mitigation of ocean deoxygenation, including mitigation of loss arising due to climate change.
4 New possibilities from green hydrogen production?
As noted, Stigebrandt and Andersson (2022) estimated that direct injection of pure oxygen was about 10 × more costly as a mitigation measure than artificial downwelling, mainly due to the price of pure oxygen. The cost of oxygen production and its transportation is likely a key factor that has limited use of pure O2 injection to applications that are either highly localized (reservoirs, harbors) or of high economic value (e.g., aquaculture pens). However, the cost and availability of oxygen may be about to change drastically as a result of rapid development of decarbonized energy systems and fuels including growing interest in use of renewable energy to produce green hydrogen and the use of ammonia as a fuel. Both industrial processes result in production of oxygen as a by-product (from electrolysis used in H2 production and air separation used in the Haber–Bosch process). It is likely that both industries will develop preferentially in coastal areas, where renewable energy (e.g., wind) is abundant and transportation to remote markets is convenient (Fig. 3).
Tracer concentration 8 months (left) and 12 months (right) following injection of a few hundred grams of SF5CF3 at a depth of 250 m at the location marked by the yellow star. The corresponding vertical spreading of the tracer (not shown) was limited to no more than 60 m above and below the injection depth (figure prepared by Sam Stevens, University of British Columbia)
Again taking the LSLE and GoSL as an example, it is notable that in August 2022, the Prime Minister of Canada and the Chancellor of Germany chose the small town of Stephenville on the southwest coast of Newfoundland (Fig. 1) as the location for signing a bilateral agreement on green hydrogen development (Natural Resources Canada 2022). This is also where World Energy GH2 Inc. plans to build up to three wind-powered hydrogen generation and ammonia production plants. According to an environmental assessment (EA) registration filed with the Government of Newfoundland and Labrador (World Energy GH2 Inc. 2022), the first plant will include a 0.5-gigawatt (GW) green hydrogen/ammonia production facility in Stephenville, together with nearby construction of up to 164 turbines for 1 GW of wind power generation. The project envisions converting most of the hydrogen to ammonia (NH3), for easier transportation. The amount of O2 that would be produced, assuming maximum operating efficiency, is ca. 5 × 105 tonnes (O2) per year although actual production may only be about half of that due to variability of wind power. At present, the company expects that “the plant will emit oxygen to the atmosphere” although the O2 by-product would be “captured as a value stream” if a market can be found. It is notable that more O2 will be produced by the first of the three proposed hydrogen/ammonia plants than our estimated rate of O2 depletion from the entire GoSL and LSLE 150 m (2 × 105 tonnes (O2) per year; see Section 2 and Fig. 4).
The location of the proposed plant is immediately adjacent to where subsurface water flows into the Gulf of St. Lawrence from the Atlantic Ocean. In October 2021, three of us (DW, WN, TT) initiated an experiment to investigate subsurface circulation pathways and timescales for these Atlantic-derived waters in order to better understand and model ongoing deoxygenation. To this end, we injected a few hundred grams of the inert, non-toxic tracer compound (SF5CF3) at a depth of 250 m, at a location about 130 km distant from the location of the proposed hydrogen/ammonia plants (see star on Fig. 1). The tracer is biogeochemically inert and follows the mixing and spreading of subsurface water within the Gulf of St. Lawrence in a manner similar to dissolved oxygen, but without a loss term due to respiration and sediment oxygen demand. We measured the tracer distribution in June 2022 and again in October 2022 and the measured distributions are shown in Fig. 3. (A full description of initial results of the tracer experiment, including evaluation of spreading velocities and horizontal and vertical eddy diffusivities based on fitting the tracer distribution to a simple model, will be published elsewhere.)
The tracer injection location was selected based on the expected presence of a landward current transporting Atlantic waters westward, into the GoSL and towards the LSLE. The tracer experiment was initiated before we were aware of plans for hydrogen/ammonia plants, so that the close proximity of our tracer injection was serendipitous. Nevertheless, the observed spreading of the tracer confirms that artificially injected oxygen would be transported by the subsurface circulation, within a few years, towards regions of the GoSL and LSLE that are facing an elevated risk of hypoxia. In contrast to the tracer, artificially injected oxygen would be subject to losses within the water column and to sediments as with naturally supplied oxygen, but despite these ongoing losses the extra input would raise dissolved oxygen levels throughout the deeper layers.
The implication is that artificial addition of oxygen into subsurface waters close to where it is to be produced from hydrogen/ammonia plant(s) has potential to mitigate future deoxygenation throughout the GoSL, including within the already hypoxic LSLE, 700 km “downstream.” The capacity of the facility, especially if additional plants are built, may even be sufficient to progressively reduce the extent and intensity of the low oxygen conditions that have developed over the past 90 years.
This may represent an attractive, economical use of “waste” O2 that might otherwise be vented to the atmosphere, and has potentially major benefits in terms of the protection or even restoration of marine biodiversity in the face of a growing threat from climate change.
5 An urgent need to address knowledge gaps
The situation in the Lower Estuary and Gulf of St. Lawrence highlights the sudden confluence of (a) increasing risk to ocean ecosystems from hypoxia driven by climate change; and (b) potential for increased availability of large quantities of pure oxygen in proximity to threatened regions of the coastal ocean. This confluence of circumstances is likely to occur elsewhere (e.g. Baltic Sea; other major estuarine systems of North America; coastal waters off China) so that proposals from industry to use oxygen to remediate hypoxia are likely to emerge, independently, all over the world in coming years. However, to date there has been little to no research conducted on methods, impacts, costs, and effectiveness of using pure oxygen to mitigate or remediate large regions of the ocean that are increasingly affected by deoxygenation. Further, policymakers, including most agencies (national and international) responsible for regulating placement of materials and wastes in the ocean (e.g., the London Convention/London Protocol), appear not to have considered this possibility. Taken together, this implies that there is an urgent need for broad-based research that can address a number of emerging questions, including:
Engineering questions
Including how and where should oxygen be introduced? Methods for oxygenation have been reviewed by Singleton and Little (2006) and include side stream supersaturation (SSS; where water is pumped to the surface, aerated, and reinjected at depth), air-lift systems, submerged downflow contact oxygenation (SDCO; such as a Speece Cone), and use of bubble-plume diffusers. (Membrane equilibration may be another option but appears untested to date.) However, Singleton and Little (2006) considered only oxygenation of shallow water environments such as lakes and reservoirs.
No aeration system has been designed, developed, or tested for the depths and scales considered here. Systems using SSS seem unlikely to be useful for deep water due to the water pumping involved. A detailed review of the long-term effectiveness of a Speece Cone in a reservoir (Horne et al. 2019) involved a device installed at only 25-m depth and used 6–13 tonnes of O2 per day, which is two orders of magnitude smaller than the quantities discussed above. Efficiency of oxygenation using submerged systems will be enhanced by high hydrostatic pressures that increase the amounts of oxygen that can be dissolved. However, key considerations for deepwater installation, in addition to costs, include understanding near-field dynamics (both vertical and horizontal) of oxygenated plumes and the preferred configuration and depth of injection to avoid artificial mixing or sediment disturbance. The carbon footprint of the oxygen delivery process should also be evaluated, although given the close connection with renewable energy generation, we expect that can be limited. The use of pure oxygen instead of air and the initial undersaturation of O2 in subsurface waters both reduce the risk of gas bubble disease in fish (which is often a consequence of N2 supersaturation). However, designs must ensure that near-field oxygen levels remain below levels that might cause O2 supersaturation within organisms that might swim from depth to the surface. This, in turn, will require information on subsea flows and daily/seasonally variable, temperature-dependent oxygen exchange capacities near the injection point.
Such considerations argue strongly for small-scale pilot studies where different injection technologies can be tested in concert with physical, chemical, and biological monitoring and modelling of near-field effects, over extended periods (months/years). Given the pace of green hydrogen development worldwide, these studies should commence now at suitable sites.
Questions concerning mitigation potential (effectiveness) and downstream risks
The rate and extent of far-field dispersion of injected oxygen, on scales of order hundreds of km (horizontal) and 100 m (vertical), will be critical to assessment of the effectiveness of mitigation, in the GoSL and elsewhere. Here, tracer studies such as shown in Fig. 3, combined with numerical modelling and oceanographic surveys, can provide key information.
Mitigation is not necessarily free of risk, which could include changes to redox-related cycling of nutrients. In an environment where low-oxygen waters and anaerobic surface sediments are already present, feedbacks could include decreased denitrification (which might increase eutrophication) or increased production of the greenhouse gas N2O. In environments where oxygen is decreasing due to climate and circulation change, such risks may not be major; however, they should be considered carefully in advance of any deployment.
We note that oxygen injection can mitigate circulation-induced oxygen loss, but will not prevent concomitant warming of subsurface waters which also impacts ecosystems. The specific risk of hypoxia, which represents a “tipping point” for marine biodiversity, may justify such a “single-target” mitigation approach; however, combined effects of reoxygenation under warmer conditions, on biogeochemical cycles and ecosystems, will also require consideration.
Effective monitoring systems for oxygen and ecological response, and methods for outcome assessment, will need to be designed and developed prior to large-scale deployment of oxygen injection. Such systems do not exist presently but are likely feasible (and economical) if based on advanced, in situ ocean measurement technology (chemical sensors, robotics, environmental DNA, acoustic and animal telemetry technologies), coupled with ocean biogeochemical modelling. Baseline monitoring with such systems should be tested and put in place prior to implementation in order that outcomes can be measured.
Finally, even if climate change is resulting in unprecedentedly fast changes in water properties including hypoxia, even faster change might result from a sudden halt in artificial oxygen injection. Changes in the economic situation, failure of infrastructure, or a drop in the demand for hydrogen could result in such a halt. It is therefore essential to assess potential effects of such a shut-down on water conditions.
Questions concerning ethics, costs, and regulations
Article 2 (Prevention of harm) of the United Nations’ “Declaration of Ethical Principles in Relation to Climate Change” (UNESCO 2017) asserts that. “States and all actors should take appropriate measures within their powers to: …. anticipate, avoid or minimize harm, wherever it might emerge, from climate change, as well as from climate mitigation and adaptation policies and actions….” This implies strongly that there is an ethical responsibility to mitigate ocean deoxygenation, if it is possible to do so.
Article 3 (Precautionary approach) further states: “Where there are threats of serious or irreversible harm, a lack of full scientific certainty should not be used as a reason for postponing cost-effective measures to anticipate, prevent or minimize the causes of climate change and mitigate its adverse effects.” Given the negative implications of deoxygenation and increased hypoxia for marine biodiversity, and the potential of new sources of oxygen to enable mitigation, the precautionary approach implies there is an ethical imperative to explore the issue further and implement, if possible. Ignoring the threat and doing nothing is not ethically acceptable.
Mitigation will, obviously, cost money and someone, or some organization(s), will have to pay costs of infrastructure, operations, monitoring, outcome assessment, etc. This may be a stumbling block.
Measures to protect marine biodiversity from impacts of climate change, or other global-scale threats, are notably rare, and frameworks for financing, outcome assessment, etc., do not exist except where a direct connection to climate change mitigation can be demonstrated (e.g., marine carbon dioxide removal; ocean-based nature-based solutions to climate change). For such cases, the availability of carbon credits has led to rapid exploration of schemes that can generate co-benefits of carbon sequestration and ecosystem restoration (e.g., so-called blue carbon). For situations such as that discussed here, where mitigation of climate change impacts does not necessarily alter greenhouse gas budgets, financing options are less obvious, especially as the actors with the power to mitigate (e.g., energy companies) are not connected directly to the sectors of society that might benefit economically (e.g., fishing, marine tourism or other ocean-related industries). As a result, use of biodiversity credits or offsets to finance mitigation, which is gaining increased attention with some forms of land-based development, is not directly applicable to the marine situation. We suggest that the question of how to finance mitigation of large-scale marine biodiversity loss due to climate change, including as a result of deoxygenation, requires urgent attention from policymakers, the conservation community, and marine industries.
Questions concerning regulation and assessment?
The experience with biodiversity credits and offsets on land, and developing experience with marine carbon dioxide removal, argues strongly for establishment of effective, economical monitoring and verification approaches in order that outcomes can be measured. Further, given that there are risks associated with any intervention into the ocean’s biogeochemical systems, some form of regulation and approval process will be required. Regional, national, and international regulatory agencies may not yet be prepared to address mitigation of deoxygenation, which appears likely to emerge suddenly, and is also likely to involve multiple jurisdictions. The Baltic Marine Environment Protection Commission, also known as the Helsinki Commission (HELCOM), is an example of an organization that may have developed relevant tools and experience to regulate/measure at the international level for a specific region. The London Convention/London Protocol may be able to play a regulatory role at a broader geographic scale, but has likely not had to consider this type of mitigation before. There are a number of important questions concerning governance and monitoring responsibilities that will need to be addressed in the very near future, and that could benefit from an early international exchange of relevant experience.
6 Concluding remarks
Based on the example from the Lower Estuary and Gulf of St. Lawrence, we expect there to be rapid development of interest in the use of oxygen produced from coastal green hydrogen plants for mitigation of ocean deoxygenation. This has potential to address a major, climate-related risk to marine biodiversity.
There is an ethical responsibility to actively consider this mitigation pathway, especially given that current approaches to protection of marine biodiversity (e.g., marine protected areas), while they may increase resilience of marine ecosystems, are likely to be relatively ineffectual in the face of a threat such as climate-driven oxygen loss.
However, there are major knowledge gaps and a lack of suitable frameworks for evaluation and implementation of this emerging option, so that we reiterate the conclusions of Conley et al. (2009) from an earlier time when large-scale engineering solutions to Baltic Sea hypoxia were being considered. They noted that “science must continue to be an integral part of any remediation effort” and that “experimental approaches and modeling, both on the land and in the sea, in addition to diverse expert scientific advice are required for effective solutions.” Most of all they noted that “scientists, engineers and regulators must work together.”
We believe it is now urgent for engineers, ocean scientists, and policymakers to recognize and act on this mitigation potential which is likely to emerge in different countries over the next few years. A coordinated research effort should be focussed immediately on this topic in order that the potential of the green hydrogen industry to mitigate a major impact of climate change on marine biodiversity can be harnessed effectively, and unintended negative consequences avoided.
Data availability
Oxygen data shown in Figs. 1 and 2 are available from the sources given in the caption to Fig. 1. Tracer data presented in Fig. 3 are preliminary and still being finalized together with additional data that will be collected during summer 2023. The complete dataset will be submitted to the Canadian Integrated Ocean Observing System when finalized. For now, these data will be made available upon reasonable request to the authors.
References
Atamanchuk D, Palter J, Palevsky H, Le Bras I, Koelling J, Nicholson D (2022) Linking oxygen and carbon uptake with the meridional overturning circulation using a transport mooring array. Oceanography 34(4). https://doi.org/10.5670/oceanog.2021.supplement.02-03
Bange HW, Freing A, Kock A, Löscher CR (2010) Marine pathways to nitrous oxide. In: Smith K (ed) Nitrous oxide and climate change, 1st edn. Routledge, pp 40–66
Bates NR, Johnson RJ (2020) Acceleration of ocean warming, salinification, deoxygenation and acidification in the surface subtropical North Atlantic Ocean. Commun Earth Environ 1(1):33. https://doi.org/10.1038/s43247-020-00030-5
Beghoura H, Gorgues T, Fransner F, Auger PA, Memery L (2023) Contrasting responses of the ocean’s oxygen minimum zones to artificial re-oxygenation. Environ Res Lett 18:084012. https://doi.org/10.1088/1748-9326/ace0cd
Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP, Gehlen M, Halloran P, Heinze C, Ilyina T, Séférian R, Tjiputra J, Vichi M (2013) Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10(10):6225–6245. https://doi.org/10.5194/bg-10-6225-2013
Brandt P, Banyte D, Dengler M, Didwischus SH, Fischer T, Greatbatch RJ, Hahn J, Kanzow T, Karstensen J, Körtzinger A, Krahmann G, Schmidtko S, Stramma L, Tanhua T, Visbeck M (2015) On the role of circulation and mixing in the ventilation of oxygen minimum zones with a focus on the eastern tropical North Atlantic. Biogeosciences 12:489–512. https://doi.org/10.5194/gb-12-489-2015
Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K, Jacinto GS, Limburg KE, Montes I, Naqvi SWA, Pitcher GC, Rabalais NN, Roman MR, Rose KA, Seibel BA, Telszewski M, Yasuhara M, Zhang J (2018) Declining oxygen in the global ocean and coastal waters. Science 359(6371):eaam7240. https://doi.org/10.1126/science.aam7240
Chabot D, Claireaux G (2008) Environmental hypoxia as a metabolic constraint on fish: the case of Atlantic cod, Gadus morhua. Mar Pollut Bull 57(6–12):287–294. https://doi.org/10.1016/j.marpolbul.2008.04.001
Claret M, Galbraith ED, Palter JB, Bianchi D, Fennel K, Gilbert D, Dunne JP (2018) Rapid coastal deoxygenation due to ocean circulation shift in the northwest Atlantic. Nat Clim Chang 8(10):868–872. https://doi.org/10.1038/s41558-018-0263-1
Conley DJ, Björck S et al (2009) Hypoxia-related processes in the Baltic Sea. Environ Sci Technol 43(10):3412–3420. https://doi.org/10.1021/es802762a
D’Amours D (1993) The distribution of cod (Gadus morhua) in relation to temperature and oxygen level in the Gulf of St. Lawrence. Fish Oceanogr 2(1):24–29. https://doi.org/10.1111/j.1365-2419.1993.tb00009.x
Department of Fisheries and Oceans Canada (2010) Synopsis of the social, economic, and cultural overview of the Gulf of St. Lawrence, Oceans, Habitat and Species at Risk Publication Series, https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/343191.pdf
Department of Fisheries and Oceans Canada (2018), BioChem: database of biological and chemical oceanographic data, retrieved from http://www.dfo-mpo.gc.ca/science/data-donnees/biochem/index-eng.html
Devine L, Kennedy MKL, St-Pierre I, Lafleur C, Ouellet M, Bond S (2014) BioChem: the Fisheries and Oceans Canada database for biological and chemical data. Can Tech Rep Fish Aquat Scie 3037:40
Diaz RJ, Rosenberg R (1995) Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr Mar Biol Annu Rev 33:245–303
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(5891):926–929. https://doi.org/10.1126/science.1156401
Dupont-Prinet A, Vagner M, Chabot D, Audet C (2013a) Impact of hypoxia on the metabolism of Greenland halibut (Reinhardtius hippoglossoides). Can J Fish Aquat Sci 70(3):461–469. https://doi.org/10.1139/cjfas-2012-0327
Dupont-Prinet A, Pillet M, Chabot D, Hansen T, Tremblay R, Audet C (2013b) Northern shrimp (Pandalus borealis) oxygen consumption and metabolic enzyme activities are severely constrained by hypoxia in the Estuary and Gulf of St. Lawrence. J Exp Mar Biol Ecol 448:298–307. https://doi.org/10.1016/j.jembe.2013.07.019
Genovesi L, De Vernal A, Thibodeau B, Hillaire-Marcel C, Mucci A, Gilbert D (2011) Recent changes in bottom water oxygenation and temperature in the Gulf of St. Lawrence: micropaleontological and geochemical evidence. Limnol Oceanogr 56(4):1319–1329. https://doi.org/10.4319/lo.2011.56.4.1319
Gerling AB, Browne RG, Gantzer PA, Mobley MH, Little JC, Carey CC (2014) First report of the successful operation of a side stream supersaturation hypolimnetic oxygenation system in a eutrophic, shallow reservoir. Water Res 67:129–143. https://doi.org/10.1016/j.watres.2014.09.002
Gilbert D, Sundby B, Gobeil C, Mucci A, Tremblay GH (2005) A seventy-two-year record of diminishing deep-water oxygen in the St. Lawrence estuary: the northwest Atlantic connection. Limnol Oceanogr 50(5):1654–1666. https://doi.org/10.4319/lo.2005.50.5.1654
Gilbert D, Chabot D, Archambault P, Rondeau B, Hébert S (2007) Appauvrissement en oxygène dans les eaux profondes du Saint-Laurent marin: causes possibles et impacts écologiques. Naturaliste Can 131:67–75
Gilbert D, Rabalais NN, Diaz RJ, Zhang J (2010) Evidence for greater oxygen decline rates in the coastal ocean than in the open ocean. Biogeosciences 7(7):2283–2296. https://doi.org/10.5194/bg-7-2283-2010
Horne AJ, Jung R, Lai H, Faisst B, Beutel M (2019) Hypolimnetic oxygenation 2: oxygen dynamics in a large reservoir with submerged down-flow contact oxygenation (Speece cone). Lake Reservoir Manage 35(3):323–337. https://doi.org/10.1080/10402381.2019.1648612
Ingall E, Jahnke R (1994) Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim Cosmochim Acta 58(11):2571–2575. https://doi.org/10.1016/0016-7037(94)90033-7
Jutras M, Dufour CO, Mucci A, Cyr F, Gilbert D (2020) Temporal changes in the causes of the observed oxygen decline in the St. Lawrence Estuary. J Geophys Res: Oceans 125(12):e2020JC016577. https://doi.org/10.1029/2020JC016577
Jutras M, Mucci A, Chaillou G, Nesbitt WA, Wallace DWR (2023) Temporal and spatial evolution of bottom-water hypoxia in the St Lawrence estuarine system. Biogeosciences 20(4):839–849. https://doi.org/10.5194/bg-20-839-2023
Kalvelage T, Lavik G, Lam P, Contreras S, Arteaga L, Löscher CR, Oschlies A, Paulmier A, Stramma L, Kuypers MM (2013) Nitrogen cycling driven by organic matter export in the South Pacific oxygen minimum zone. Nat Geosci 6(3):228–234. https://doi.org/10.1038/ngeo1739
Keeling RF, Manning A (2013) Studies of recent changes in atmospheric O2 content. Treat Geochem 5:385–404. https://doi.org/10.1016/B978-0-08-095975-7.00420-4
Koelling J, Wallace DWR, Send U, Karstensen J (2017) Intense oceanic uptake of oxygen during 2014–2015 winter convection in the Labrador Sea. Geophys Res Lett 44(15):7855–7864. https://doi.org/10.1002/2017GL073933
Koweek DA, García-Sánchez C, Brodrick PG, Gassett P, Caldeira K (2020) Evaluating hypoxia alleviation through induced downwelling. Sci Total Environ 719:137334. https://doi.org/10.1016/j.scitotenv.2020.137334
Kullenberg G (1970) On the oxygen deficit in the Baltic deep water. Tellus 22(3):357–357. https://doi.org/10.3402/tellusa.v22i3.10229
Liu S, Zhao L, Xiao C, Fan W, Cai Y, Pan Y, Chen Y (2020) Review of artificial downwelling for mitigating hypoxia in coastal waters. Water 12(10):2846. https://doi.org/10.3390/w12102846
Natural Resources Canada (2022) Canada and Germany Sign Agreement to Enhance German Energy Security with Clean Canadian Hydrogen. http://www.canada.ca/en/natural-resources-canada/news/2022/08/canada-and-germany-sign-agreement-to-enhance-german-energy-security-with-clean-canadian-hydrogen.html
Oschlies A, Brandt P, Stramma L, Schmidtko S (2018) Drivers and mechanisms of ocean deoxygenation. Nat Geosci 11(7):467–473. https://doi.org/10.1038/s41561-018-0152-2
Penn JL, Deutsch C (2022) Avoiding ocean mass extinction from climate warming. Science 376(6592):524–526. https://doi.org/10.1126/science.abe9039
Petersen LH, Gamperl AK (2010) Effect of acute and chronic hypoxia on the swimming performance, metabolic capacity and cardiac function of Atlantic cod (Gadus morhua). J Exp Biol 213(5):808–819. https://doi.org/10.1242/jeb.033746
Rabalais NN, Diaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J (2010) Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7(2):585–619. https://doi.org/10.5194/bg-7-585-2010
Raiswell R, Canfield DE (2012) The iron biogeochemical cycle past and present. Geochem Perspect 1(1):1–2
Saba VS, Griffies SM, Anderson WG, Winton M, Alexander MA, Delworth TL, Hare JA, Harrison MJ, Vecchi GA, Zhang R (2016) Enhanced warming of the Northwest Atlantic Ocean under climate change. J Geophys Res: Oceans 121(1):118–132. https://doi.org/10.1002/2015JC011346
Sampaio E, Santos C, Rosa IC, Ferreira V, Pörtner H, Duarte CM, Levin LA, Rosa R (2021) Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat Ecol Evol 5:311–321. https://doi.org/10.1038/s41559-020-01370-3
Schmidtko S, Stramma L, Visbeck M (2017) Decline in global oceanic oxygen content during the past five decades. Nature 542(7641):335–339. https://doi.org/10.1038/nature21399
Scholz F, McManus J, Mix AC, Hensen C, Schneider RR (2014) The impact of ocean deoxygenation on iron release from continental margin sediments. Nat Geosci 7(6):433–437. https://doi.org/10.1038/ngeo2162
Singleton VL, Little JC (2006) Designing hypolimnetic aeration and oxygenation systems− a review. Environ Sci Technol 40(24):7512–7520. https://doi.org/10.1021/es060069s
Stigebrandt A, Andersson A (2020) The eutrophication of the Baltic Sea has been boosted and perpetuated by a major internal phosphorus source. Front Mar Sci 7:572994. https://doi.org/10.3389/fmars.2020.57299
Stigebrandt A, Andersson A (2022) Improving oxygen conditions in periodically stagnant basins using sea-based measures-illustrated by hypothetical applications to the By Fjord, Sweden. Cont Shelf Res 244:104806. https://doi.org/10.1016/j.csr.2022.104806
Stigebrandt A, Liljebladh B, De Brabandere L, Forth M, Granmo Å, Hall P, Hammer J, Hansson D, Kononets M, Magnusson M, Norén F, Rahm L, Treusch AH, Viktorsson L (2015) An experiment with forced oxygenation of the deepwater of the anoxic By Fjord, western Sweden. Ambio 44:42–54. https://doi.org/10.1007/s13280-014-0524-9
Stramma L, Schmidtko S (2021) Spatial and temporal variability of oceanic oxygen changes and underlying trends. Atmos Ocean 59(2):122–132. https://doi.org/10.1080/07055900.2021.1905601
Stramma L, Johnson GC, Sprintall J, Mohrholz V (2008) Expanding oxygen-minimum zones in the tropical oceans. Science 320(5876):655–658. https://doi.org/10.1126/science.1153847
Stramma L, Prince E, Schmidtko S, Luo J, Hoolihan JP, Visbeck M, Wallace DWR, Brandt P, Körtzinger A (2012) Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat Clim Chang 2:33–37. https://doi.org/10.1038/nclimate1304
Stramma L, Visbeck M, Brandt P, Tanhua T, Wallace DWR (2009) Deoxygenation in the oxygen minimum zone of the eastern tropical North Atlantic. Geophys Res Lett 36(20). https://doi.org/10.1029/2009GL039593
Sundby B, Gobeil C, Silverberg N, Mucci A (1992) The phosphorus cycle in coastal marine sediments. Limnol Oceanogr 37(6):1129–1145. https://doi.org/10.4319/lo.1992.37.6.1129
Thibodeau B, de Vernal A, Mucci A (2006) Recent eutrophication and consequent hypoxia in the bottom waters of the Lower St. Lawrence Estuary: micropaleontological and geochemical evidence. Mar Geol 231(1–4):37–50. https://doi.org/10.1016/j.margeo.2006.05.010
UNESCO General Conference, 37th, (2017) Declaration of ethical principles in relation to climate change, https://unesdoc.unesco.org/ark:/48223/pf0000260889.page=127
Wolf MK, Hamme RC, Gilbert D, Yashayaev I, Thierry V (2018) Oxygen saturation surrounding deep water formation events in the Labrador Sea from Argo-O2 data. Global Biogeochem Cycles 32(4):635–653. https://doi.org/10.1002/2017GB005829
World Energy GH2 Inc (2022) Project Nujio’qonik GH2 Environmental Assessment Registration, https://www.gov.nl.ca/ecc/files/2202-Registration-Document.pdf
Wright LD, Greene GC, Maa JPY, Siddiqi S (1992) Passive artificial ventilation of hypoxic estuarine benthic environments: a feasibility study. J Coastal Res 8(1):134–152
Acknowledgements
The deliberate tracer experiment (TReX) in the Gulf of St. Lawrence whose results are shown in Fig. 3 was jointly supported by the Marine Environmental Observation, Prediction and Response Network of Centres of Excellence (MEOPAR; https://www.meopar.ca) and the Réseau Québec maritime (https://www.rqm.quebec/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wallace, D.W.R., Jutras, M., Nesbitt, W.A. et al. Can green hydrogen production be used to mitigate ocean deoxygenation? A scenario from the Gulf of St. Lawrence. Mitig Adapt Strateg Glob Change 28, 56 (2023). https://doi.org/10.1007/s11027-023-10094-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11027-023-10094-1