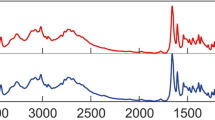
To ensure the uniformity of measurements and metrological traceability of measurement results in state regulation of ensuring the uniformity of measurements, including in healthcare and pharmaceuticals, reference materials of composition, properties and structure of substances are needed. Currently, the Federal Information Fund of the Russian Federation has approximately 9000 reference materials, among which only about 30 reference materials are intended for use in pharmaceuticals and medicine. This amount is clearly insufficient for full metrological support of these areas. This article describes the main stages and results of research and development of reference materials of starting pharmaceutical substances amphotericin B, natamycin, olivomycin A. The main substances of the created reference materials are identified. Mass fractions of related compounds, residual organic solvents and inorganic impurities (iron cations and heavy metals) were determined. The results of the work were used to confirm the reference material types of the starting pharmaceutical substances amphotericin B, natamycin, olivomycin A.




Similar content being viewed by others
References
VIM 3. International Vocabulary of Metrology. Basic and General Concepts and Associated Terms, 3rd ed. [Russian translation], Mendeleev VNIIM, BelGIM, NPO Professional, St. Petersburg (2010), https://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf, acc. Jan. 10, 2020.
Yashpal S. Chhonker, Yarra Durga Prasad, Hardik Chandasana, et al., Int. J. Biol. Macromol., 72, 1451–1458 (2015), DOI: https://doi.org/10.1016/j.ijbiomac.2014.10.014.
Lyes Mehenni, Malika Lahiani-Skiba, Guy Ladam, et al., Pharmaceutics, 10, No. 4, 235 (2018), DOI: https://doi.org/10.3390/pharmaceutics10040235.
A. N. Tevyashova, “Olivomycin A – antitumor antibiotic of the aureol acid group,” Khim.-Farm. Zh., 50, No. 7, 3–5 (2016), DOI: https://doi.org/10.30906/0023-1134-2016-50-7-3-8.
Houssam M. Atta, Sh. M. Selim, and Mona S. Zayed, J. Amer. Sci., 8 (2), 469–475 (2012), www.americanscience.org, acc. Jan. 10, 2020.
P. Sowinski, J. Pawlak, and E. Borowski, Magn. Reson. Chem., 30, 275–279 (1992), DOI: https://doi.org/10.1002/mrc.1260300402.
J. M. Brown and P. J. Sidebottom, Tetrahedron, 37, 1421–1428 (1981).
L. Volpon and J.-M. Lancelin, Eur. J. Biochem., 269, 4533–4541 (2002), DOI: https://doi.org/10.1046/j.1432-1033.2002.03147.x.
Y. Yoshimura, M. Koenuma, K. Matsumoto, et al., J. Antibiot. (Tokyo), 41, 53–67 (1988), DOI: https://doi.org/10.7164/antibiotics.41.53.
P. Grundt, T. Findeisen, E. Miethke, et al., J. Clin. Microbiol., 50 (5), 1727–9 (2012), DOI: https://doi.org/10.1128/JCM.00047-12.
Vishal Diwan, Ashok J. Tamhankar, Rakesh K. Khandal, et al., BMC Public Health, 10 (1), 414 (2010), DOI: https://doi.org/10.1186/1471-2458-10-414.
Prakash Srinivasan, Ajit K. Sarmah, Merilyn Manley-Harris, and Alistair L. Wilkins, J. Environ. Sci. Heal. A, 47 (13), 2120–2132 (2012), DOI: https://doi.org/10.1080/10934529.2012.696005.
Z. Tzouganaki and M. Koupparis, Mediter. J. Chem., 6 (4), 133–141 (2017), DOI: https://doi.org/10.13171/mjc64/01706211420-tzouganaki.
P. N. Patil and Sh. Jacob, Int. J. Pharm. Sci. Res., 3 (1) (2012), DOI: https://doi.org/10.15373/22501991/APR2014/98.
Yuexi Yang, Chen Huan, Xianrui Liang, et al., Molecules, 24 (21), 3962, (2019) DOI: https://doi.org/10.3390/molecules24213962.
Rajamani Lakshminarayanan, Radhakrishnan Sridhar, Xian Jun Loh, et al., Int. J. Nanomed., 9 (1), 439–458, (2014), DOI: https://doi.org/10.2147/IJN.S58487.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel’naya Tekhnika, No. 4, pp. 66–71, March, 2020.
Rights and permissions
About this article
Cite this article
Kuliabina, E.V., Tevyashova, A.N., Solov’eva, S.E. et al. Reference Materials of Composition of Biologically Active Substances. Meas Tech 63, 325–331 (2020). https://doi.org/10.1007/s11018-020-01790-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-020-01790-4