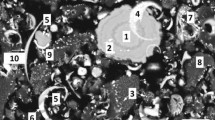
In this study, we investigate the effect of low-temperature leaching modes to leach copper and silver from secondary copper electrolytic refining slime with sulfuric acid solution.
Promising fields for intensification of the leaching process of secondary copper electrolytic refining slime , namely preliminary oxidizing roasting and microwave processing, are determined. The influence of firing temperature (500°C–800°C) on the characteristics of calcines and recovery of copper and silver into sulfuric acid solution in the presence of hydrogen peroxide are studied. The formation of new phases in a temperature range of 500°C–800°C, namely silver-containing copper hydroxosulfate, lead- containing barite, and copper oxide, at firing temperature more than 700°C is revealed. The maximum copper and silver recovery into the leaching solution from the slime is 96.40% and 84.10%, respectively. Regression equations of the effect of microwave treatment power (300–530 W) and duration (5–9 min) on copper and silver recovery into the leaching solution are determined via mathematical planning of the experiment. In the studied factor-variation intervals, the maximum recovery of copper and silver into the leaching solution is 96.74% and 35.72%, respectively.









Similar content being viewed by others
Notes
The authors express their gratitude to the Director General of PROMTEHRECIKLING Nikolay Vasilyevich Taran for providing a sample for research.
References
A. M. Chekmarev, Solvometallurgy is a Promising Direction in the Metallurgy of Rare and Non-Ferrous Metals [in Russian], Atomenergoizdat, Moscow (2004); ISBN 5-98532-002-2.
S. O. Vydysh, E. V. Bogatyreva, Zh. N. Galieva, and A. A. Semenov, “Study on copper and silver collective recovery from secondary copper electrolytic refining slimes. Part 1,” Metallurg, No. 5, 89–97 (2023).
S. A. Mastyugin, N. A. Volkova, S. S. Naboichenko, and M. A. Lastochkina, Slime from Electrolytic Refining of Copper [in Russian], UrFU, Yekaterinburg (2013). ISBN 978-5-321-02296-2.
Yu. B. Lyapishchev, “State-of-the-art of processing of electrolytic slime of copper production,” Zapiski Gorn. Inst., 167, No. 2, 245–247 (2006).
S. A. Mastyugin and S. S. Naboychenko, “Processing of copper electrolyte slime: technology evolution,” Izv. Vyssh. Ucheb. Zav. Tsvetn. Metallurg., No. 5, 15–21 (2012).
G. Lui, Y. Wu, A. Tang, D. Pan, and B. Li, “Recovery of scattered and precious metals from copper anode slime by hydrometallurgy: A review,” Hydrometallurgy, 197, 105460 (2020).
S. Rao, Y. Liu, D. Wang, H. Cao, W. Zhu, R. Yang, L. Duan, and Z. Liu, “Pressure leaching of selenium and tellurium from scrap copper anode slimes in sulfuric acid-oxygen media,” J. Clean. Prod., 278, 123989 (2021).
R. K. Amankwah and G. Ofori-Sarpong, “Microwave heating of gold ores for enhanced grindability and cyanide amenability,” Miner. Eng., 24, 541–544 (2010).
N. S. Ratnikova and P. V. Pankratiev, “Improvement of efficiency of extracting gold and silver from pyrite concentrates using microwave technology,” Vest. MGTU im. G. I. Nosova, 17, No. 4, 4–9 (2018).
Z. Guo, F. Li, Q. Zhang, G. Su, J. Chang, and H. Zhou, “Microwave roasting characteristics of cuprous chloride residue from zinc hydrometallurgy,” Crystals, 12, No. 1, 116 (2022).
M. Gupta and W. W. Leong, Microwaves and Metals, Wiley (2007); ISBN 978-0-470-82272-2.
J. W. Walkiewicz, G. Kazonich, and S. L. McGill, “Microwave heating characteristics of selected minerals and compounds,” Miner. Metallurg. Process., 5, 39–42 (1988).
M. Levas, M. Kovacova, A. Zubrik, M. Kovacova, and S. Dolinska, “The application of microwave energy in mineral processing – a review,” Acta Montan. Slovaca, 16, No. 2, 137–148 (2011).
H. Zeng, P. Liu, Y. Hong, K. Yang, and L. Zhang, “Hg/Se/PbSO4 Recovery by microwave-intensified HgSe pyrolysis from toxic acid mud,” Metals, 12, 1038 (2022).
Z. Peng and J. -Y. Hwang, “Microwave-assisted metallurgy,” Int. Mater. Rev., 60, No. 1, 30–63 (2015).
Yu. P. Adler, E. V. Markova, and Yu. V. Granovsky, Planning an Experiment in the Search for Optimal Conditions [in Russian], Nauka, Moscow (1976).
R. A. Lidin, V. A. Molochko, and L. L. Andreeva, Reactions of Inorganic Substances [in Russian], Reference Book, Drofa, Moscow (2007); ISBN 978-5-358-01303-2.
R. A. Lidin, L. L. Andreeva, and V. A. Molochko, Constants of Inorganic Substances [in Russian], Reference Book, Drofa, Moscow (2008); ISBN 978-5-358-04347-3.
A. A. Ravdel and A. M. Ponomareva, Brief Reference Book of Physical and Chemical Quantities [in Russian], Ivan Fedorov, Saint-Petersburg (2003); ISBN5-8194-00071-2.
A. B. Lebed, S. S. Naboychenko, and V. A. Shunin, Production of Selenium and Tellurium at Uralelektromed [in Russian], Textbook, Izdatel’stvo Ural Univ., Yekaterinburg (2015); ISBN 978-5-358-01303-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 67, No. 6, pp. 107–114, June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vydysh, S.O., Bogatyreva, E.V., Galieva, Z.N. et al. Collective Recovery of Copper and Silver from Secondary Copper Electrolytic Refining Slimes. Part 2. Metallurgist 67, 857–871 (2023). https://doi.org/10.1007/s11015-023-01573-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-023-01573-6