Abstract
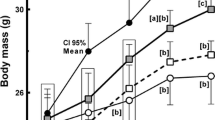
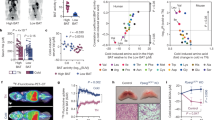
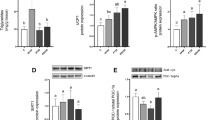
Dietary interventions that modulate the brown adipose tissue (BAT) thermogenic activity could represent a promising therapy for metabolic disorders. In order to examine if dietary walnuts intake regulates the expression of BAT thermogenic markers levels in healthy and metabolically challenged (fructose fed) animals, rats were initially divided into the control and fructose-fed groups. After nine weeks, these groups were subdivided into the one kept on the original regimens and the other supplemented with walnuts. High-fructose diet resulted in an increased relative BAT mass and no change in UCP1 content, while the walnut supplementation increased the amount of UCP1 in BAT, but did not affect 5-HT, NA, DHPG content and DHPG/NA ratio regardless of the diet. Moreover, the CD36 levels were increased following the walnut consumption, unlike FATP1, GLUT1, GLUT4, and glycogen content which remained unchanged. Additionally, the BAT levels of activated IR and Akt were not affected by walnut consumption, while ERK signaling was decreased. Overall, we found that walnut consumption increased UCP1 and CD36 content in the BAT of both control and metabolically challenged rats, suggesting that FFAs represent the BAT preferred substrate under the previously described circumstances. This further implies that incorporating walnuts into the everyday diet may help to alleviate some symptoms of the metabolic disorder.







Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Bargut TCL, Silva-e-Silva ACAG, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB (2016) Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur J Nutr 55. https://doi.org/10.1007/s00394-015-0834-0
Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PLSM, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17. https://doi.org/10.1038/nm.2297
Betz MJ, Enerbäck S (2018) Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol 14:77–87. https://doi.org/10.1038/nrendo.2017.132
Bolling BW, McKay DL, Blumberg JB (2010) The phytochemical composition and antioxidant actions of tree nuts. Asia Pac J Clin Nutr 19:117–123. https://doi.org/10.6133/apjcn.2010.19.1.16
Brenna JT, Salem N, Sinclair AJ, Cunnane SC (2009) α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fat Acids 80. https://doi.org/10.1016/j.plefa.2009.01.004
Calderon-Dominguez M, Mir JF, Fucho R, Weber M, Serra D, Herrero L (2016) Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 5:98–118. https://doi.org/10.1080/21623945.2015.1122857
Castro-Barquero S, Ruiz-León AM, Sierra-Pérez M, Estruch R, Casas R (2020) Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients 12:2983. https://doi.org/10.3390/nu12102983
Chan AML, Ng AMH, Mohd Yunus MH, Idrus RBH, Law JX, Yazid MD, Chin KY, Shamsuddin SA, Lokanathan Y (2021) Recent developments in rodent models of high-fructose diet-induced metabolic syndrome: a systematic review. Nutrients 13:2497. https://doi.org/10.3390/nu13082497
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A, Kolodny GM, Kahn CR (2009) Identification and importance of Brown Adipose tissue in adult humans. N Engl J Med 360:1509–1517. https://doi.org/10.1056/nejmoa0810780
Dong M, Lin J, Lim W, Jin W, Lee HJ (2018) Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front Med 12:130–138. https://doi.org/10.1007/s11684-017-0555-2
Farkas V, Kelenyi G, Sandor A (1999) A dramatic accumulation of glycogen in the brown adipose tissue of rats following recovery from cold exposure. Arch Biochem Biophys 365. https://doi.org/10.1006/abbi.1999.1157
Fedorenko A, Lishko PV, Kirichok Y (2012) Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151:400–413. https://doi.org/10.1016/j.cell.2012.09.010
Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB (2001) Stimulation of Lipolysis and hormone-sensitive lipase via the Extracellular Signal-regulated kinase pathway. J Biol Chem 276. https://doi.org/10.1074/jbc.m104436200
Gunawan S, Aulia A, Soetikno V (2021) Development of rat metabolic syndrome models: a review. Vet World 14:1774–1783. https://doi.org/10.14202/vetworld.2021.1774-1783
Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312. https://doi.org/10.1126/science.1123965
Hao Q, Yadav R, Basse AL, Petersen S, Sonne SB, Rasmussen S, Zhu Q, Lu Z, Wang J, Audouze K, Gupta R, Madsen L, Kristiansen K, Hansen JB (2015) Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. Am J Physiol - Endocrinol Metab 308. https://doi.org/10.1152/ajpendo.00277. 2014
Herz CT, Kulterer OC, Prager M, Schmöltzer C, Langer FB, Prager G, Marculescu R, Kautzky-Willer A, Hacker M, Haug AR, Kiefer FW (2022) Active brown adipose tissue is Associated with a healthier metabolic phenotype in obesity. Diabetes 71. https://doi.org/10.2337/db21-0475
Huber KR, Wödl H, Robubi A, Hauser A, Schrattbauer K, Krugluger W (2016) In Vitro Culture of Human Brown adipocytes: effects of Fructose. Int J Life Sci Med Res 6:1–8. https://doi.org/10.5963/LSMR0601001
Hwang HJ, Liu Y, Kim HS, Lee H, Lim Y, Park H (2019) Daily walnut intake improves metabolic syndrome status and increases circulating adiponectin levels: Randomized controlled crossover trial. Nutr Res Pract 13. https://doi.org/10.4162/nrp.2019.13.2.105
Jakus PB, Sandor A, Janaky T, Farkas V (2008) Cooperation between BAT and WAT of rats in thermogenesis in response to cold, and the mechanism of glycogen accumulation in BAT during reacclimation. J Lipid Res 49. https://doi.org/10.1194/jlr.m700316-jlr200
Jatkar A, Kurland IJ, Judex S (2017) Diets high in Fat or Fructose differentially modulate bone health and lipid metabolism. Calcif Tissue Int 100. https://doi.org/10.1007/s00223-016-0205-8
Jung SM, Doxsey WG, Le J, Haley JA, Mazuecos L, Luciano AK, Li H, Jang C, Guertin DA (2021) In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep 36:109459. https://doi.org/10.1016/j.celrep.2021.109459
Kawada T, Kayahashi S, Hida Y, Koga KJ, Nadachi Y, Fushiki T (1998) Fish (Bonito) Oil Supplementation enhances the expression of uncoupling protein in Brown Adipose tissue of rat. J Agric Food Chem 46. https://doi.org/10.1021/jf9711000
Khedoe PPSJ, Hoeke G, Kooijman S, Dijk W, Buijs JT, Kersten S, Havekes LM, Hiemstra PS, Berbée JFP, Boon MR, Rensen PCN (2015) Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res 56. https://doi.org/10.1194/jlr.m052746
Khitan Z, Kim DH (2013) Fructose: a key factor in the development of metabolic syndrome and hypertension. J Nutr Metab 2013:1–12. https://doi.org/10.1155/2013/682673
Kim SH, Plutzky J (2016) Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J 40:12–21. https://doi.org/10.4093/dmj.2016.40.1.12
Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y, Takahashi N, Kawada T (2015) Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci Rep 5. https://doi.org/10.1038/srep18013
Kuipers EN, Held NM, Panhuis W (2019) in het, Modder M, Ruppert PMM, Kersten S, Kooijman S, Guigas B, Houtkooper RH, Rensen PCN, Boon MR A single day of high-fat diet feeding induces lipid accumulation and insulin resistance in brown adipose tissue in mice. Am J Physiol - Endocrinol Metab 317. https://doi.org/10.1152/ajpendo.00123.2019
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-Phenol Reagent. J Biol Cemistry 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM (2004) Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr 55:171–178. https://doi.org/10.1080/09637480410001725175
McNeill BT, Morton NM, Stimson RH (2020) Substrate utilization by Brown Adipose tissue: what’s Hot and what’s not? Front Endocrinol 11:1–8. https://doi.org/10.3389/fendo.2020.571659
Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, Malan A (1997) Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Int J Obes 21. https://doi.org/10.1038/sj.ijo.0800500
Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122. https://doi.org/10.117 2/jci60433
Pandareesh MD, Chauhan V, Chauhan A (2018) Walnut Supplementation in the Diet reduces oxidative damage and improves antioxidant status in transgenic mouse model of Alzheimer’s Disease. J Alzheimers Dis JAD 64:1295–1305. https://doi.org/10.3233/jad-180361
Petrović-Oggiano G, Debeljak-Martačić J, Ranković S, Pokimica B, Mirić A, Glibetić M, Popović T (2020) The effect of walnut consumption on n-3 fatty acid profile of healthy people living in a non-mediterranean west balkan country, a small scale randomized study. Nutrients 12:192. https://doi.org/10.3390/nu12010192
Postic C, Leturque A, Printz RL, Maulard P, Loizeau M, Granner DK, Girard J (1994) Development and regulation of glucose transporter and hexokinase expression in rat. Am J Physiol - Endocrinol Metab 266:E548–E559. https://doi.org/10.1152/ajpendo.1994.266.4.e548
Putri M, Syamsunarno MRAA, Iso T, Yamaguchi A, Hanaoka H, Sunaga H, Koitabashi N, Matsui H, Yamazaki C, Kameo S, Tsushima Y, Yokoyama T, Koyama H, Abumrad NA, Kurabayashi M (2015) CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem Biophys Res Commun 457. https://doi.org/10.1016/j.bbrc.201412.124
Rasouli M, Ostovar-Ravari A, Shokri-Afra H (2014) Characterization and improvement of phenol-sulfuric acid microassay for glucose-based glycogen. Eur Rev Med Pharmacol Sci 18:2020–2024
Rasouli M, Shokri-Afra H, Ostovar-Ravari A (2015) A new protocol for separation of acid soluble and insoluble fractions from total glycogen and simultaneous measurements. Eur Rev Med Pharmacol Sci 19:1785–1789
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661. https://doi.org/10.1096/fj.07-9574lsf
Richard G, Blondin DP, Syed SA, Rossi L, Fontes ME, Fortin M, Phoenix S, Frisch F, Dubreuil S, Guérin B, Turcotte ÉE, Lepage M, Surette MG, Schertzer JD, Steinberg GR, Morrison KM, Carpentier AC (2022) High-fructose feeding suppresses cold-stimulated brown adipose tissue glucose uptake independently of changes in thermogenesis and the gut microbiome. Cell Rep Med 3:100742. https://doi.org/10.1016/j.xcrm.2022.100742
Roberts-Toler C, O’Neill BT, Cypess AM (2015) Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. https://doi.org/10.1002/oby.21134. Obesity 23
Ros E, Br (2015) J Nutr 113:S111–S120. https://doi.org/10.1017/s0007114514003924
Rothwell NJ, Stock MJ (1979) A role for brown adipose tissue in diet-induced thermogenesis. Nature 281. https://doi.org/10.1038/281031a0
Saito M, Matsushita M, Yoneshiro T, Okamatsu-Ogura Y (2020) Brown Adipose tissue, Diet-Induced thermogenesis, and thermogenic food ingredients: from mice to men. Front Endocrinol 11:222. https://doi.org/10.3389/fendo.2020.00222
Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, Jackson R, Rumore D, Xue B, Shi H, Gavrilova O, Yu L (2017) Lipolysis in Brown adipocytes is not essential for Cold-Induced thermogenesis in mice. Cell Metab 26. https://doi.org/10.1016/j.cmet.2017.09.002
Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123. https://doi.org/10.1172/jci62308
Stanisic J, Ivkovic T, Romic S, Zec M, Culafic T, Stojiljkovic M, Koricanac G (2020) Beneficial effect of walnuts on vascular tone is associated with akt signalling, voltage-dependent calcium channel LTCC and ATP-sensitive potassium channel Kv1.2. Int J Food Sci Nutr 21:1–11. https://doi.org/10.1080/09637486.2020.1796931
Townsend K, Tseng Y-H (2014) Brown Fat fuel utilization and thermogenesis. Trends Endocrinol Metab 25:168–177. https://doi.org/10.1016/j.tem.2013.12.004
Wade G, McGahee A, Ntambi JM, Simcox J (2021) Lipid Transport in brown adipocyte thermogenesis. Front Physiol 12:787535. https://doi.org/10.3389/fphys.2021.787535
Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206:2049–2057. https://doi.org/10.1242/jeb.00241
Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A, Stahl A (2006) Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55. https://doi.org/10.2337/db06-0749
Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E (2000) Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med 132:538–546. https://doi.org/10.7326/0003-4819-132-7-200004040-00005
Zec MM, Krga I, Takić M, Debeljak-Martačić J, Korićanac G, Ranković S, Popović T, Pantelić M, Glibetic M (2020) Walnut Consumption induces tissue-specific Omega-6/Omega-3 decrease in high-fructose-Fed Wistar rats. ACS Omega 5:28136–28145. https://doi.org/10.1021/acsomega.0c03784
Funding
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, under the contract numbers 451-03-65/2024-03/ 200178 and 451-03-66/2024-03/ 200178.
Author information
Authors and Affiliations
Contributions
TJ conceived and designed the study. Material preparation, analysis and data collection were performed by DJ and TD. NJ performed the HPLC analysis. TJ and TD interpreted the data. IL collected tissue and contributed to the interpretation of the results. DJ wrote the Methods and Results section, rest of the first manuscript draft was written by TD. TJ, IL, AR, and PV reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dakic, T., Jeremic, D., Lakic, I. et al. Walnut supplementation increases levels of UCP1 and CD36 in brown adipose tissue independently of diet type. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04981-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04981-7