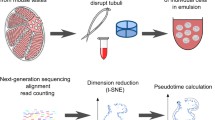
Abstract
Spermatogenesis, a key part of the spermiation process, is regulated by a combination of key cells, such as primordial germ cells, spermatogonial stem cells, and somatic cells, such as Sertoli cells. Abnormal spermatogenesis can lead to azoospermia, testicular tumors, and other diseases related to male infertility. The application of single-cell RNA sequencing (scRNA-seq) technology in male reproduction is gradually increasing with its unique insight into deep mining and analysis. The data cover different periods of neonatal, prepubertal, pubertal, and adult stages. Different types of male infertility diseases including obstructive and non-obstructive azoospermia (NOA), Klinefelter Syndrome (KS), Sertoli Cell Only Syndrome (SCOS), and testicular tumors are also covered. We briefly review the principles and application of scRNA-seq and summarize the research results and application directions in spermatogenesis in different periods and pathological states. Moreover, we discuss the challenges of applying this technology in male reproduction and the prospects of combining it with other technologies.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created in this study.
Abbreviations
- APLN/APJ:
-
Apelin peptide/Apelin-receptor
- DMR:
-
Differentially methylated region
- GCNA:
-
Germ-cell nuclear antigen
- hPGC:
-
Human primordial germ cell
- KS:
-
Klinefelter syndrome
- MMLV:
-
Moloney murine leukemia virus
- NOA:
-
Non-obstructive azoospermia
- OA:
-
Obstructive azoospermia
- PGC:
-
Primordial germ cell
- SCOS:
-
Sertoli cell only syndrome
- scRNA-seq:
-
Single-cell RNA sequencing
- SNP:
-
Single nucleotide polymorphism
- SSC:
-
Spermatogonial stem cell
- T2DM:
-
Type 2 diabetes mellitus
- TF:
-
Transcription factor
- TSO:
-
Template-switching oligo
- UMI:
-
Unique molecular indentifier
References
Carrageta DF, Guerra-Carvalho B, Spadella MA, Yeste M, Oliveira PF, Alves MG (2022) Animal models of male reproductive ageing to study testosterone production and spermatogenesis. Rev Endocr Metab Disord 23:1341–1360. https://doi.org/10.1007/s11154-022-09726-9
Shima Y (2022) Functional importance of mini-puberty in spermatogenic stem cell formation. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2022.907989
Clermont Y (1966) Spermatogenesis in Man. Fertil. Steril.
Griswold MD (2016) Spermatogenesis: the commitment to meiosis. Physiol Rev 96:1–17. https://doi.org/10.1152/physrev.00013.2015
Ishikura Y, Ohta H, Sato T, Murase Y, Yabuta Y, Kojima Y, Yamashiro C, Nakamura T, Yamamoto T, Ogawa T, Saitou M (2021) In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell. https://doi.org/10.1016/j.stem.2021.08.005
Hess RA, Renato de Franca L (2008) Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636:1–15. https://doi.org/10.1007/978-0-387-09597-4_1
Yao M, Qu H, Han Y, Cheng CY, Xiao X (2022) Kinesins in mammalian spermatogenesis and germ cell transport. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2022.837542
Washburn RL, Hibler T, Kaur G, Dufour JM (2022) Sertoli cell immune regulation: a double-edged sword. Front Immunol. https://doi.org/10.3389/fimmu.2022.913502
Nie X, Munyoki SK, Sukhwani M, Schmid N, Missel A, Emery BR, DonorConnect SJB, Mayerhofer A, Orwig KE, Aston KI, Hotaling JM, Cairns BR, Guo J (2022) Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev Cell. https://doi.org/10.1016/j.devcel.2022.04.004
Chen P, Zirkin BR, Chen H (2020) Stem leydig cells in the adult testis: characterization, regulation and potential applications. Endocr Rev 41:22–32. https://doi.org/10.1210/endrev/bnz013
Choy KHK, Chan SY, Lam W, Jin J, Zheng T, Law TYS, Yu SS, Wang W, Li L, Xie G, Yim HCH, Chen H, Fok EKL (2022) The repertoire of testicular extracellular vesicle cargoes and their involvement in inter-compartmental communication associated with spermatogenesis. BMC Biol 20:78. https://doi.org/10.1186/s12915-022-01268-5
Guan X, Ji M, Wen X, Huang F, Zhao X, Chen D, Shao J, Wang J, Xie J, Tian J, Lin H, Duan P, Zirkin BR, Su Z, Chen H (2022) Single-cell RNA sequencing of adult rat testes after Leydig cell elimination and restoration. Sci Data 9:106. https://doi.org/10.1038/s41597-022-01225-5
Chen L, Li Y, Zhu L, Jin H, Kang X, Feng Z (2023) Single-cell RNA sequencing in the context of neuropathic pain: progress, challenges, and prospects. Transl Res 251:96–103. https://doi.org/10.1016/j.trsl.2022.07.004
Hoft SG, Pherson MD, DiPaolo RJ (2022) Discovering immune-mediated mechanisms of gastric carcinogenesis through single-cell RNA sequencing. Front Immunol. https://doi.org/10.3389/fimmu.2022.902017
Jovic D, Liang X, Zeng H, Lin L, Xu F, Luo Y (2022) Single-cell RNA sequencing technologies and applications: A brief overview. Clin Transl Med. https://doi.org/10.1002/ctm2.694
Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA (2009) mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods 6:377–382. https://doi.org/10.1038/nmeth.1315
Hicks SC, Townes FW, Teng M, Irizarry RA (2018) Missing data and technical variability in single-cell RNA-sequencing experiments. Biostatistics 19:562–578. https://doi.org/10.1093/biostatistics/kxx053
Zhang QY, Ho DW, Tsui YM, Ng IO (2022) Single-cell transcriptomics of liver cancer: hype or insights? Cell Mol Gastroenterol Hepatol 14:513–525. https://doi.org/10.1016/j.jcmgh.2022.04.014
Ramskold D, Luo SJ, Wang YC, Li R, Deng QL, Faridani OR, Daniels GA, Khrebtukova I, Loring JF, Laurent LC, Schroth GP, Sandberg R (2012) Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat Biotechnol 30:777–782. https://doi.org/10.1038/nbt.2282
Zhang YJ, Wang D, Peng M, Tang L, Ouyang JW, Xiong F, Guo C, Tang YY, Zhou YJ, Liao QJ, Wu X, Wang H, Yu JJ, Li Y, Li XL, Li GY, Zeng ZY, Tan YX, Xiong W (2021) Single-cell RNA sequencing in cancer research. J Exp Clin Cancer Res. https://doi.org/10.1186/s13046-021-01874-1
Picelli S (2017) Single-cell RNA-sequencing: The future of genome biology is now. RNA Biol 14:637–650. https://doi.org/10.1080/15476286.2016.1201618
Goetz JJ, Trimarchi JM (2012) Transcriptome sequencing of single cells with Smart-Seq. Nat Biotechnol 30:763–765. https://doi.org/10.1038/nbt.2325
Picelli S, Bjorklund AK, Faridani OR, Sagasser S, Winberg G, Sandberg R (2013) Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods 10:1096–1098. https://doi.org/10.1038/nmeth.2639
Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R (2014) Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 9:171–181. https://doi.org/10.1038/nprot.2014.006
Picelli S (2019) Full-Length Single-Cell RNA Sequencing with Smart-seq2. In: Proserpio V (ed) Single Cell Methods: Sequencing and Proteomics, pp. 25–44
Hagemann-Jensen M, Ziegenhain C, Chen P, Ramskold D, Hendriks GJ, Larsson AJM, Faridani OR, Sandberg R (2020) Single-cell RNA counting at allele and isoform resolution using Smart-seq3. Nat Biotechnol 38:708. https://doi.org/10.1038/s41587-020-0497-0
Hagemann-Jensen M, Ziegenhain C and Sandberg R Scalable single-cell RNA sequencing from full transcripts with Smart-seq3xpress. Nature Biotechnology. doi: https://doi.org/10.1038/s41587-022-01311-4
Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA (2015) Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161:1202–1214. https://doi.org/10.1016/j.cell.2015.05.002
Schekman R (2010) Editorial Expression of Concern for multiple articles. Proc Natl Acad Sci U S A 107:6551. https://doi.org/10.1073/pnas.1003210107
Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, Gregory MT, Shuga J, Montesclaros L, Underwood JG, Masquelier DA, Nishimura SY, Schnall-Levin M, Wyatt PW, Hindson CM, Bharadwaj R, Wong A, Ness KD, Beppu LW, Deeg HJ, McFarland C, Loeb KR, Valente WJ, Ericson NG, Stevens EA, Radich JP, Mikkelsen TS, Hindson BJ, Bielas JH (2017) Massively parallel digital transcriptional profiling of single cells. Nat Commun 8:14049. https://doi.org/10.1038/ncomms14049
Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW (2015) Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161:1187–1201. https://doi.org/10.1016/j.cell.2015.04.044
Bageritz J, Raddi G (2019) Single-cell RNA sequencing with drop-seq. Methods Mol Biol 1979:73–85. https://doi.org/10.1007/978-1-4939-9240-9_6
Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC, Teichmann SA (2015) The technology and biology of single-cell RNA sequencing. Mol Cell 58:610–620. https://doi.org/10.1016/j.molcel.2015.04.005
Zilionis R, Nainys J, Veres A, Savova V, Zemmour D, Klein AM, Mazutis L (2017) Single-cell barcoding and sequencing using droplet microfluidics. Nat Protoc 12:44–73. https://doi.org/10.1038/nprot.2016.154
Han Y, Wang D, Peng L, Huang T, He X, Wang J, Ou C (2022) Single-cell sequencing: a promising approach for uncovering the mechanisms of tumor metastasis. J Hematol Oncol 15:59. https://doi.org/10.1186/s13045-022-01280-w
Kedar N, Natarajan, (2019) Single-cell tagged reverse transcription (STRT-Seq). Methods Mol Biol 1979:133–153
Islam S, Kjallquist U, Moliner A, Zajac P, Fan JB, Lonnerberg P, Linnarsson S (2011) Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res 21:1160–1167. https://doi.org/10.1101/gr.110882.110
Gla B, Qian GB, Sz A, Bo YA (2020) Probing infectious disease by single-cell RNA sequencing: Progresses and perspectives - ScienceDirect. Comput Struct Biotechnol J 18:2962–2971
Kivioja T, Vhrautio A, Karlsson K, Bonke M, Taipale J (2011) Counting absolute number of molecules using unique molecular identifiers. Nat Prec. https://doi.org/10.1038/npre.2011.5903.1
Tang DT, Plessy C, Salimullah M, Suzuki AM, Calligaris R, Gustincich S, Carninci P (2013) Suppression of artifacts and barcode bias in high-throughput transcriptome analyses utilizing template switching. Nucleic Acids Res. https://doi.org/10.1093/nar/gks1128
Fan HC, Fu GK, Fodor SP (2015) Expression profiling. Combinatorial labeling of single cells for gene expression cytometry. Science. https://doi.org/10.1126/science.1258367
Gierahn TM, Wadsworth MH 2nd, Hughes TK, Bryson BD, Butler A, Satija R, Fortune S, Love JC, Shalek AK (2017) Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat Methods 14:395–398. https://doi.org/10.1038/nmeth.4179
Han Q, Bagheri N, Bradshaw EM, Hafler DA, Lauffenburger DA, Love JC (2012) Polyfunctional responses by human T cells result from sequential release of cytokines. Proc Natl Acad Sci U S A 109:1607–1612. https://doi.org/10.1073/pnas.1117194109
Yamanaka YJ, Berger CT, Sips M, Cheney PC, Alter G, Love JC (2012) Single-cell analysis of the dynamics and functional outcomes of interactions between human natural killer cells and target cells. Integr Biol (Camb) 4:1175–1184. https://doi.org/10.1039/c2ib20167d
Zhang X, Li T, Liu F, Chen Y, Yao J, Li Z, Huang Y, Wang J (2019) Comparative analysis of droplet-based ultra-high-throughput single-cell RNA-Seq systems. Mol Cell. https://doi.org/10.1016/j.molcel.2018.10.020
Ziegenhain C, Vieth B, Parekh S, Reinius B, Guillaumet-Adkins A, Smets M, Leonhardt H, Heyn H, Hellmann I, Enard W (2017) Comparative analysis of single-cell RNA sequencing methods. Mol Cell. https://doi.org/10.1016/j.molcel.2017.01.023
Wang X, He Y, Zhang Q, Ren X, Zhang Z (2021) Direct comparative analyses of 10X genomics chromium and smart-seq2. Genom Proteomics Bioinform 19:253–266. https://doi.org/10.1016/j.gpb.2020.02.005
Zhang M, Zou Y, Xu X, Zhang X, Gao M, Song J, Huang P, Chen Q, Zhu Z, Lin W, Zare RN, Yang C (2020) Highly parallel and efficient single cell mRNA sequencing with paired picoliter chambers. Nat Commun 11:2118. https://doi.org/10.1038/s41467-020-15765-0
Tang WWC, Kobayashi T, Irie N, Dietmann S, Surani MA (2016) Specification and epigenetic programming of the human germ line. Nat Rev Genet 17:585–600. https://doi.org/10.1038/nrg.2016.88
Hancock GV, Wamaitha SE, Peretz L, Clark AT (2021) Mammalian primordial germ cell specification. Development. https://doi.org/10.1242/dev.189217
Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y, Wang W, Li R, Yan J, Zhi X, Zhang Y, Jin H, Zhang W, Hou Y, Zhu P, Li J, Zhang L, Liu S, Ren Y, Zhu X, Wen L, Gao YQ, Tang F, Qiao J (2015) The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell 161:1437–1452. https://doi.org/10.1016/j.cell.2015.05.015
Pierson Smela M, Sybirna A, Wong FCK, Surani MA (2019) Testing the role of SOX15 in human primordial germ cell fate. Wellcome open research 4:122–122. https://doi.org/10.12688/wellcomeopenres.15381.1
Chen D, Sun N, Hou L, Kim R, Faith J, Aslanyan M, Tao Y, Zheng Y, Fu J, Liu W, Kellis M, Clark A (2019) Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep 29:4568. https://doi.org/10.1016/j.celrep.2019.11.083
Tang WWC, Castillo-Venzor A, Gruhn WH, Kobayashi T, Penfold CA, Morgan MD, Sun D, Irie N, Surani MA (2022) Sequential enhancer state remodelling defines human germline competence and specification. Nat Cell Biol 24:448–460. https://doi.org/10.1038/s41556-022-00878-z
Sharma S, Wistuba J, Pock T, Schlatt S, Neuhaus N (2019) Spermatogonial stem cells: updates from specification to clinical relevance. Hum Reprod Update 25:275–297. https://doi.org/10.1093/humupd/dmz006
Tan K, Wilkinson MF (2020) A single-cell view of spermatogonial stem cells. Curr Opin Cell Biol 67:71–78. https://doi.org/10.1016/j.ceb.2020.07.005
Guo J, Grow EJ, Yi C, Mlcochova H, Maher GJ, Lindskog C, Murphy PJ, Wike CL, Carrell DT, Goriely A, Hotaling JM, Cairns BR (2017) Chromatin and single-cell RNA-Seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell. https://doi.org/10.1016/j.stem.2017.09.003
Guo J, Grow EJ, Mlcochova H, Maher GJ, Lindskog C, Nie X, Guo Y, Takei Y, Yun J, Cai L, Kim R, Carrell DT, Goriely A, Hotaling JM, Cairns BR (2018) The adult human testis transcriptional cell atlas. Cell Res 28:1141–1157. https://doi.org/10.1038/s41422-018-0099-2
Hermann BP, Cheng K, Singh A, Roa-De la Cruz L, Mutoji KN, Chen IC, Gildersleeve H, Lehle JD, Mayo M, Westernstroer B, Law NC, Oatley MJ, Velte EK, Niedenberger BA, Fritze D, Silber S, Geyer CB, Oatley JM, McCarrey JR (2018) The mammalian spermatogenesis single-cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep 25:1650. https://doi.org/10.1016/j.celrep.2018.10.026
Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, Hsieh TC, Rabah R, Hammoud SS, Vicini E, Wilkinson MF (2019) The neonatal and adult human testis defined at the single-cell level. Cell Rep 26:1501. https://doi.org/10.1016/j.celrep.2019.01.045
Law NC, Oatley MJ, Oatley JM (2019) Developmental kinetics and transcriptome dynamics of stem cell specification in the spermatogenic lineage. Nat Commun. https://doi.org/10.1038/s41467-019-10596-0
Guo J, Sosa E, Chitiashvili T, Nie X, Rojas EJ, Oliver E, Plath K, Hotaling JM, Stukenborg J-B, Clark AT, Cairns BR, DonorConnect, (2021) Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 28:764. https://doi.org/10.1016/j.stem.2020.12.004
Xia B, Yan Y, Baron M, Wagner F, Barkley D, Chiodin M, Kim SY, Keefe DL, Alukal JP, Boeke JD, Yanai I (2020) Widespread transcriptional scanning in the testis modulates gene evolution rates. Cell. https://doi.org/10.1016/j.cell.2019.12.015
La H, Yoo H, Lee EJ, Thang NX, Choi HJ, Oh J, Park JH, Hong K (2021) Insights from the applications of single-cell transcriptomic analysis in germ cell development and reproductive medicine. Int J Mol Sci. https://doi.org/10.3390/ijms22020823
Guo J, Nie X, Giebler M, Mlcochova H, Wang Y, Grow EJ, DonorConnect KR, Tharmalingam M, Matilionyte G, Lindskog C, Carrell DT, Mitchell RT, Goriely A, Hotaling JM, Cairns BR (2020) The dynamic transcriptional cell atlas of testis development during human puberty. Cell Stem Cell. https://doi.org/10.1016/j.stem.2019.12.005
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R (2018) Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36:411–420. https://doi.org/10.1038/nbt.4096
Zhao L, Yao C, Xing X, Jing T, Li P, Zhu Z, Yang C, Zhai J, Tian R, Chen H, Luo J, Liu N, Deng Z, Lin X, Li N, Fang J, Sun J, Wang C, Zhou Z, Li Z (2020) Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun 11:5683. https://doi.org/10.1038/s41467-020-19414-4
Santiago J, Silva JV, Alves MG, Oliveira PF, Fardilha M (2019) Testicular aging: an overview of ultrastructural, cellular, and molecular alterations. J Gerontol A Biol Sci Med Sci 74:860–871. https://doi.org/10.1093/gerona/gly082
Knight BE, Kozlowski N, Havelin J, King T, Crocker SJ, Young EE, Baumbauer KM (2019) TIMP-1 attenuates the development of inflammatory pain through MMP-dependent and receptor-mediated cell signaling mechanisms. Front Mol Neurosci 12:220. https://doi.org/10.3389/fnmol.2019.00220
Oliveira PF, Alves MG (2015) The Sertoli Cell at a Glance. In: Oliveira PF, Alves MG (eds) Sertoli Cell Metabolism and Spermatogenesis. Springer, Cham, pp 3–13
Salomon TB, Hackenhaar FS, Almeida AC, Schuller AK, Gil Alabarse PV, Ehrenbrink G, Benfato MS (2013) Oxidative stress in testis of animals during aging with and without reproductive activity. Exp Gerontol 48:940–946. https://doi.org/10.1016/j.exger.2013.06.010
Cao L, Leers-Sucheta S, Azhar S (2004) Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol 88:61–67. https://doi.org/10.1016/j.jsbmb.2003.10.007
Niederberger C (2017) Re: clinical, genetic, biochemical, and testicular biopsy findings among 1213 men evaluated for infertility. J Urol 198:468–470. https://doi.org/10.1016/j.juro.2017.06.043
Skakkebaek NE (1969) Two types of tubules containing only Sertoli cells in adults with Klinefelter’s syndrome. Nature 223:643–645. https://doi.org/10.1038/223643a0
Tiepolo L, Zuffardi O (1976) Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet 34:119–124. https://doi.org/10.1007/BF00278879
Wang M, Xu Y, Zhang Y, Chen Y, Chang G, An G, Yang X, Zheng C, Zhao J, Liu Z, Wang D, Miao K, Rao S, Dai M, Wang D, Zhao XY (2021) Deciphering the autophagy regulatory network via single-cell transcriptome analysis reveals a requirement for autophagy homeostasis in spermatogenesis. Theranostics 11:5010–5027. https://doi.org/10.7150/thno.55645
Wang M, Liu X, Chang G, Chen Y, An G, Yan L, Gao S, Xu Y, Cui Y, Dong J, Chen Y, Fan X, Hu Y, Song K, Zhu X, Gao Y, Yao Z, Bian S, Hou Y, Lu J, Wang R, Fan Y, Lian Y, Tang W, Wang Y, Liu J, Zhao L, Wang L, Liu Z, Yuan R, Shi Y, Hu B, Ren X, Tang F, Zhao XY, Qiao J (2018) Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. https://doi.org/10.1016/j.stem.2018.08.007
Chen S, An G, Wang H, Wu X, Ping P, Hu L, Chen Y, Fan J, Cheng CY, Sun F (2022) Human obstructive (postvasectomy) and nonobstructive azoospermia - Insights from scRNA-Seq and transcriptome analysis. Genes Dis 9:766–776. https://doi.org/10.1016/j.gendis.2020.09.004
Di Persio S, Leitao E, Woste M, Tekath T, Cremers JF, Dugas M, Li X, Horste MZ, G, Kliesch S, Laurentino S, Neuhaus N and Horsthemke B, (2021) Whole-genome methylation analysis of testicular germ cells from cryptozoospermic men points to recurrent and functionally relevant DNA methylation changes. Clin Epigenetics 13:160. https://doi.org/10.1186/s13148-021-01144-z
Bhargava V, Goldstein CD, Russell L, Xu L, Ahmed M, Li W, Casey A, Servage K, Kollipara R, Picciarelli Z, Kittler R, Yatsenko A, Carmell M, Orth K, Amatruda JF, Yanowitz JL, Buszczak M (2020) GCNA preserves genome integrity and fertility across species. Dev Cell. https://doi.org/10.1016/j.devcel.2019.11.007
Dokshin GA, Davis GM, Sawle AD, Eldridge MD, Nicholls PK, Gourley TE, Romer KA, Molesworth LW, Tatnell HR, Ozturk AR, de Rooij DG, Hannon GJ, Page DC, Mello CC, Carmell MA (2020) GCNA interacts with spartan and topoisomerase II to regulate genome stability. Dev Cell. https://doi.org/10.1016/j.devcel.2019.11.006
Hardy JJ, Wyrwoll MJ, McFadden W, Malcher A, Rotte N, Pollock NC, Munyoki S, Veroli MV, Houston BJ, Xavier MJ, Kasak L, Punab M, Laan M, Kliesch S, Schlegel P, Jaffe T, Hwang K, Vukina J, Brieno-Enriquez MA, Orwig K, Yanowitz J, Buszczak M, Veltman JA, Oud M, Nagirnaja L, Olszewska M, O’Bryan MK, Conrad DF, Kurpisz M, Tuttelmann F, Yatsenko AN and Consortium G (2021) Variants in GCNA, X-linked germ-cell genome integrity gene, identified in men with primary spermatogenic failure. Hum Genet 140:1169–1182. https://doi.org/10.1007/s00439-021-02287-y
Nagirnaja L, Lopes AM, Charng WL, Miller B, Stakaitis R, Golubickaite I, Stendahl A, Luan T, Friedrich C, Mahyari E, Fadial E, Kasak L, Vigh-Conrad K, Oud MS, Xavier MJ, Cheers SR, James ER, Guo J, Jenkins TG, Riera-Escamilla A, Barros A, Carvalho F, Fernandes S, Goncalves J, Gurnett CA, Jorgensen N, Jezek D, Jungheim ES, Kliesch S, McLachlan RI, Omurtag KR, Pilatz A, Sandlow JI, Smith J, Eisenberg ML, Hotaling JM, Jarvi KA, Punab M, Rajpert-De Meyts E, Carrell DT, Krausz C, Laan M, O’Bryan MK, Schlegel PN, Tuttelmann F, Veltman JA, Almstrup K, Aston KI, Conrad DF (2022) Diverse monogenic subforms of human spermatogenic failure. Nat Commun 13:7953. https://doi.org/10.1038/s41467-022-35661-z
Klinefelter HF, Reifenstein EC, Albright F (1942) Syndrome characterized by gynecomastia, aspermatogenesis without A-Leydigism, and increased excretion of follicle-stimulating hormone1. J Clin Endocrinol Metabolism 2:615–627. https://doi.org/10.1210/jcem-2-11-615
Lanfranco F, Kamischke A, Zitzmann M and Nieschlag E (2004) Klinefelter\"s syndrome. 364:0–283.
Jacobs PA, Strong JA (1959) A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183:302–303. https://doi.org/10.1038/183302a0
Navarro-Cobos MJ, Balaton BP, Brown CJ (2020) Genes that escape from X-chromosome inactivation: Potential contributors to Klinefelter syndrome. Am J Med Genet C Semin Med Genet 184:226–238. https://doi.org/10.1002/ajmg.c.31800
Astro V, Alowaysi M, Fiacco E, Saera-Vila A, Cardona-Londono KJ, Aiese Cigliano R, Adamo A (2021) Pseudoautosomal region 1 overdosage affects the global transcriptome in iPSCs from patients with klinefelter syndrome and high-grade X chromosome aneuploidies. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2021.801597
He H, Huang T, Yu F, Chen K, Guo S, Zhang L, Tang X, Yuan X, Liu J, Zhou Y (2022) KIF2C affects sperm cell differentiation in patients with Klinefelter syndrome, as revealed by RNA-Seq and scRNA-Seq data. FEBS Open Bio 12:1465–1474. https://doi.org/10.1002/2211-5463.13446
Ritter A, Kreis NN, Louwen F, Wordeman L, Yuan J (2015) Molecular insight into the regulation and function of MCAK. Crit Rev Biochem Mol Biol 51:228–245. https://doi.org/10.1080/10409238.2016.1178705
Ems-McClung SC, Hertzer KM, Zhang X, Miller MW, Walczak CE (2007) The interplay of the N- and C-terminal domains of MCAK control microtubule depolymerization activity and spindle assembly. Mol Biol Cell 18:282–294. https://doi.org/10.1091/mbc.e06-08-0724
Santi D, De Vincentis S, Magnani E, Spaggiari G (2017) Impairment of sperm DNA methylation in male infertility: a meta-analytic study. Andrology 5:695–703. https://doi.org/10.1111/andr.12379
Laurentino S, Heckmann L, Di Persio S, Li X, Horste MZ, G, Wistuba J, Cremers JF, Gromoll J, Kliesch S, Schlatt S and Neuhaus N, (2019) High-resolution analysis of germ cells from men with sex chromosomal aneuploidies reveals normal transcriptome but impaired imprinting. Clin Epigenetics 11:127. https://doi.org/10.1186/s13148-019-0720-3
Winge SB, Soraggi S, Schierup MH, Rajpert-De Meyts E, Almstrup K (2020) Integration and reanalysis of transcriptomics and methylomics data derived from blood and testis tissue of men with 47, XXY Klinefelter syndrome indicates the primary involvement of Sertoli cells in the testicular pathogenesis. Am J Med Genet C Semin Med Genet 184:239–255. https://doi.org/10.1002/ajmg.c.31793
Mahyari E, Guo J, Lima AC, Lewinsohn DP, Stendahl AM, Vigh-Conrad KA, Nie X, Nagirnaja L, Rockweiler NB, Carrell DT, Hotaling JM, Aston KI, Conrad DF (2021) Comparative single-cell analysis of biopsies clarifies pathogenic mechanisms in Klinefelter syndrome. Am J Hum Genet 108:1924–1945. https://doi.org/10.1016/j.ajhg.2021.09.001
Panda R, Kalmady SV, Greiner R (2022) Multi-source domain adaptation techniques for mitigating batch effects: a comparative study. Front Neuroinform. https://doi.org/10.3389/fninf.2022.805117
Siniscalchi C, Di Palo A, Russo A, Potenza N (2022) The lncRNAs at X chromosome inactivation center: not just a matter of sex dosage compensation. Int J Mol Sci. https://doi.org/10.3390/ijms23020611
Ferlin A, Arredi B, Speltra E, Cazzadore C, Selice R, Garolla A, Lenzi A, Foresta C (2007) Molecular and clinical characterization of Y chromosome microdeletions in infertile men: a 10-year experience in Italy. J Clin Endocrinol Metab 92:762–770. https://doi.org/10.1210/jc.2006-1981
Mistry BV, Zhao Y, Chang TC, Yasue H, Chiba M, Oatley J, Diaz F, Liu WS (2013) Differential expression of PRAMEL1, a cancer/testis antigen, during spermatogenesis in the mouse. PLoS ONE. https://doi.org/10.1371/journal.pone.0060611
Birtle Z, Goodstadt L, Ponting C (2005) Duplication and positive selection among hominin-specific PRAME genes. BMC Genomics 6:120. https://doi.org/10.1186/1471-2164-6-120
Joshi S, Davies H, Sims LP, Levy SE, Dean J (2007) Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol 7:67. https://doi.org/10.1186/1471-213X-7-67
Wang Z, Xu X, Li JL, Palmer C, Maric D, Dean J (2019) Sertoli cell-only phenotype and scRNA-seq define PRAMEF12 as a factor essential for spermatogenesis in mice. Nat Commun 10:5196. https://doi.org/10.1038/s41467-019-13193-3
Song K, Yang X, An G, Xia X, Zhao J, Xu X, Wan C, Liu T, Zheng Y, Ren S, Wang M, Chang G, Cronin SJF, Penninger JM, Jing T, Ou X, Rao S, Liu Z, Zhao XY (2022) Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models. Nat Commun 13:7335. https://doi.org/10.1038/s41467-022-34990-3
Pierre T, Selhane F, Zareski E, Garcia C, Fizazi K, Loriot Y, Patrikidou A, Naoun N, Bernard-Tessier A, Baumert H, Lebacle C, Blanchard P, Rocher L, Balleyguier C (2022) The role of CT in the staging and follow-up of testicular tumors: baseline, recurrence and pitfalls. Cancers. https://doi.org/10.3390/cancers14163965
Moch H, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, Menon S, Raspollini MR, Rubin MA, Srigley JR, Hoon Tan P, Tickoo SK, Tsuzuki T, Turajlic S, Cree I, Netto GJ (2022) The 2022 World Health Organization classification of tumours of the Ur inary system and male genital organs-part A: renal, penile, and testic ular tumours. Eur Urol. https://doi.org/10.1016/j.eururo.2022.06.016
Znaor A, Skakkebaek N, Rajpert-De Meyts E, Kuliš T, Laversanne M, Gurney J, Sarfati D, McGlynn K, Bray F (2022) Global patterns in testicular cancer incidence and mortality in 2020. Int J Cancer 151:692–698. https://doi.org/10.1002/ijc.33999
Webster NJ, Maywald RL, Benton SM, Dawson EP, Murillo OD, LaPlante EL, Milosavljevic A, Lanza DG, Heaney JD (2021) Testicular germ cell tumors arise in the absence of sex-specific differentiation. Development. https://doi.org/10.1242/dev.197111
Mo L, Yu Z, Lv Y, Cheng J, Yan H, Lu W, Su C, Ling Q, Mo Z (2022) Single-cell RNA sequencing of metastatic testicular seminoma reveals the cellular and molecular characteristics of metastatic cell lineage. Front Oncol. https://doi.org/10.3389/fonc.2022.871489
Xu X, Liu Z, Li Y, Fan L, Wang S, Guo J, Luo Y, Bo H (2022) Single nuclear RNA sequencing highlights intra-tumoral heterogeneity and tumor microenvironment complexity in testicular embryonic rhabdomyosarcoma. J Inflamm Res 15:493–507. https://doi.org/10.2147/JIR.S343068
Kashima Y, Sakamoto Y, Kaneko K, Seki M, Suzuki Y, Suzuki A (2020) Single-cell sequencing techniques from individual to multiomics analyses. Exp Mol Med 52:1419–1427. https://doi.org/10.1038/s12276-020-00499-2
Dey SS, Kester L, Spanjaard B, Bienko M, van Oudenaarden A (2015) Integrated genome and transcriptome sequencing of the same cell. Nat Biotechnol 33:285–289. https://doi.org/10.1038/nbt.3129
Macaulay IC, Haerty W, Kumar P, Li YI, Hu TX, Teng MJ, Goolam M, Saurat N, Coupland P, Shirley LM, Smith M, Van der Aa N, Banerjee R, Ellis PD, Quail MA, Swerdlow HP, Zernicka-Goetz M, Livesey FJ, Ponting CP, Voet T (2015) G&T-seq: parallel sequencing of single-cell genomes and transcriptomes. Nat Methods 12:519–522. https://doi.org/10.1038/nmeth.3370
Han KY, Kim KT, Joung JG, Son DS, Kim YJ, Jo A, Jeon HJ, Moon HS, Yoo CE, Chung W, Eum HH, Kim S, Kim HK, Lee JE, Ahn MJ, Lee HO, Park D, Park WY (2018) SIDR: simultaneous isolation and parallel sequencing of genomic DNA and total RNA from single cells. Genome Res 28:75–87. https://doi.org/10.1101/gr.223263.117
Rodriguez-Meira A, Buck G, Clark SA, Povinelli BJ, Alcolea V, Louka E, McGowan S, Hamblin A, Sousos N, Barkas N, Giustacchini A, Psaila B, Jacobsen SEW, Thongjuea S, Mead AJ (2019) Unravelling intratumoral heterogeneity through high-sensitivity single-cell mutational analysis and parallel RNA sequencing. Mol Cell. https://doi.org/10.1016/j.molcel.2019.01.009
Bell AD, Mello CJ, Nemesh J, Brumbaugh SA, Wysoker A, McCarroll SA (2020) Insights into variation in meiosis from 31,228 human sperm genomes. Nature 583:259–264. https://doi.org/10.1038/s41586-020-2347-0
Angermueller C, Clark SJ, Lee HJ, Macaulay IC, Teng MJ, Hu TX, Krueger F, Smallwood S, Ponting CP, Voet T, Kelsey G, Stegle O, Reik W (2016) Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat Methods 13:229–232. https://doi.org/10.1038/nmeth.3728
Chen S, Lake BB, Zhang K (2019) High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat Biotechnol 37:1452–1457. https://doi.org/10.1038/s41587-019-0290-0
Zhu C, Yu M, Huang H, Juric I, Abnousi A, Hu R, Lucero J, Behrens MM, Hu M, Ren B (2019) An ultra high-throughput method for single-cell joint analysis of open chromatin and transcriptome. Nat Struct Mol Biol 26:1063–1070. https://doi.org/10.1038/s41594-019-0323-x
Liu L, Liu C, Quintero A, Wu L, Yuan Y, Wang M, Cheng M, Leng L, Xu L, Dong G, Li R, Liu Y, Wei X, Xu J, Chen X, Lu H, Chen D, Wang Q, Zhou Q, Lin X, Li G, Liu S, Wang Q, Wang H, Fink JL, Gao Z, Liu X, Hou Y, Zhu S, Yang H, Ye Y, Lin G, Chen F, Herrmann C, Eils R, Shang Z, Xu X (2019) Deconvolution of single-cell multi-omics layers reveals regulatory heterogeneity. Nat Commun 10:470. https://doi.org/10.1038/s41467-018-08205-7
Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R, Smibert P (2017) Simultaneous epitope and transcriptome measurement in single cells. Nat Methods 14:865–868. https://doi.org/10.1038/nmeth.4380
Peterson VM, Zhang KX, Kumar N, Wong J, Li L, Wilson DC, Moore R, McClanahan TK, Sadekova S, Klappenbach JA (2017) Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol 35:936–939. https://doi.org/10.1038/nbt.3973
Stahl PL, Salmen F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M, Mollbrink A, Linnarsson S, Codeluppi S, Borg A, Ponten F, Costea PI, Sahlen P, Mulder J, Bergmann O, Lundeberg J, Frisen J (2016) Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353:78–82. https://doi.org/10.1126/science.aaf2403
Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ (2019) Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363:1463–1467. https://doi.org/10.1126/science.aaw1219
Chen H, Murray E, Sinha A, Laumas A, Li J, Lesman D, Nie X, Hotaling J, Guo J, Cairns BR, Macosko EZ, Cheng CY, Chen F (2021) Dissecting mammalian spermatogenesis using spatial transcriptomics. Cell Rep. https://doi.org/10.1016/j.celrep.2021.109915
Wu H, Kirita Y, Donnelly EL, Humphreys BD (2019) Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol 30:23–32. https://doi.org/10.1681/ASN.2018090912
Slyper M, Porter CBM, Ashenberg O, Waldman J, Drokhlyansky E, Wakiro I, Smillie C, Smith-Rosario G, Wu J, Dionne D, Vigneau S, Jane-Valbuena J, Tickle TL, Napolitano S, Su MJ, Patel AG, Karlstrom A, Gritsch S, Nomura M, Waghray A, Gohil SH, Tsankov AM, Jerby-Arnon L, Cohen O, Klughammer J, Rosen Y, Gould J, Nguyen L, Hofree M, Tramontozzi PJ, Li B, Wu CJ, Izar B, Haq R, Hodi FS, Yoon CH, Hata AN, Baker SJ, Suva ML, Bueno R, Stover EH, Clay MR, Dyer MA, Collins NB, Matulonis UA, Wagle N, Johnson BE, Rotem A, Rozenblatt-Rosen O, Regev A (2020) A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors. Nat Med 26:792–802. https://doi.org/10.1038/s41591-020-0844-1
Kiselev VY, Andrews TS, Hemberg M (2019) Challenges in unsupervised clustering of single-cell RNA-seq data. Nat Rev Genet 20:273–282. https://doi.org/10.1038/s41576-018-0088-9
Hedlund E, Deng Q (2018) Single-cell RNA sequencing: Technical advancements and biological applications. Mol Aspects Med 59:36–46. https://doi.org/10.1016/j.mam.2017.07.003
Lahnemann D, Koster J, Szczurek E, McCarthy DJ, Hicks SC, Robinson MD, Vallejos CA, Campbell KR, Beerenwinkel N, Mahfouz A, Pinello L, Skums P, Stamatakis A, Attolini CS, Aparicio S, Baaijens J, Balvert M, Barbanson B, Cappuccio A, Corleone G, Dutilh BE, Florescu M, Guryev V, Holmer R, Jahn K, Lobo TJ, Keizer EM, Khatri I, Kielbasa SM, Korbel JO, Kozlov AM, Kuo TH, Lelieveldt BPF, Mandoiu II, Marioni JC, Marschall T, Molder F, Niknejad A, Raczkowski L, Reinders M, Ridder J, Saliba AE, Somarakis A, Stegle O, Theis FJ, Yang H, Zelikovsky A, McHardy AC, Raphael BJ, Shah SP, Schonhuth A (2020) Eleven grand challenges in single-cell data science. Genome Biol 21:31. https://doi.org/10.1186/s13059-020-1926-6
Imoto Y, Nakamura T, Escolar EG, Yoshiwaki M, Kojima Y, Yabuta Y, Katou Y, Yamamoto T, Hiraoka Y, Saitou M (2022) Resolution of the curse of dimensionality in single-cell RNA sequencing data analysis. Life Sci Alliance. https://doi.org/10.26508/lsa.202201591
Dal Molin A, Di Camillo B (2019) How to design a single-cell RNA-sequencing experiment: pitfalls, challenges and perspectives. Brief Bioinform 20:1384–1394. https://doi.org/10.1093/bib/bby007
Chen D, Sun N, Hou L, Kim R, Faith J, Aslanyan M, Tao Y, Zheng Y, Fu J, Liu W, Kellis M, Clark A (2019) Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep. https://doi.org/10.1016/j.celrep.2019.11.083
Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, Hsieh TC, Rabah R, Hammoud SS, Vicini E, Wilkinson MF (2019) The neonatal and adult human testis defined at the single-cell level. Cell Rep. https://doi.org/10.1016/j.celrep.2019.01.045
Guo J, Sosa E, Chitiashvili T, Nie X, Rojas EJ, Oliver E, DonorConnect PK, Hotaling JM, Stukenborg JB, Clark AT, Cairns BR (2021) Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 28:764. https://doi.org/10.1016/j.stem.2020.12.004
Han B, Yan Z, Yu S, Ge W, Li Y, Wang Y, Yang B, Shen W, Jiang H, Sun Z (2021) Infertility network and hub genes for nonobstructive azoospermia utilizing integrative analysis. Aging (Albany NY) 13:7052–7066. https://doi.org/10.18632/aging.202559
Zhang N, Wang Y, Chen Z, Ren J, Rehman A, Ahmad DW, Long D, Hou J, Zhou Y, Yang L, Ni Y, Li Y, Du C, Yu Y, Liao M (2022) Single-cell transcriptome analysis of Bisphenol A exposure reveals the key roles of the testicular microenvironment in male reproduction. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2021.112449
Zheng W, Zhang S, Jiang S, Huang Z, Chen X, Guo H, Li M, Zheng S (2021) Evaluation of immune status in testis and macrophage polarization associated with testicular damage in patients with non-obstructive azoospermia. Am J Reprod Immunol 86:e13481. https://doi.org/10.1111/aji.13481
He H, Yu F, Shen W, Chen K, Zhang L, Lou S, Zhang Q, Chen S, Yuan X, Jia X, Zhou Y (2021) The novel key genes of non-obstructive azoospermia affect spermatogenesis: transcriptomic analysis based on RNA-Seq and scRNA-Seq data. Front Genet. https://doi.org/10.3389/fgene.2021.608629
Tang XJ, Xiao QH, Wang XL, He Y, Tian YN, Xia BT, Guo Y, Huang JL, Duan P, Tan Y (2022) Single-cell transcriptomics-based study of transcriptional regulatory features in the non-obstructive azoospermia testis. Front Genet 13:875762. https://doi.org/10.3389/fgene.2022.875762
Liu X, Chen Y, Tang W, Zhang L, Chen W, Yan Z, Yuan P, Yang M, Kong S, Yan L, Qiao J (2020) Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci China Life Sci 63:1006–1015. https://doi.org/10.1007/s11427-020-1705-0
Zhou R, Lv X, Chen T, Chen Q, Tian H, Yang C, Guo W, Liu C (2021) Construction and external validation of a 5-gene random forest model to diagnose non-obstructive azoospermia based on the single-cell RNA sequencing of testicular tissue. Aging (Albany NY) 13:24219–24235. https://doi.org/10.18632/aging.203675
Funding
This work was supported by grants from the National Natural Science Foundation of China(32270912), Natural Science Foundation of Hunan Province (2021JJ41091) and College Students Innovation and Entrepreneurship Training Program of Central South University(XCX2022140).
Author information
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Ethics approval is not applicable to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, H., Wang, W., Zhou, Z. et al. Single-cell RNA sequencing technology in human spermatogenesis: Progresses and perspectives. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04840-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04840-x