Abstract
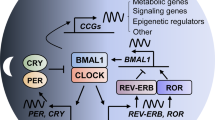
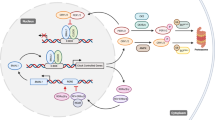
Pancreatic cancer (PC) has a very high mortality rate globally. Despite ongoing efforts, its prognosis has not improved significantly over the last two decades. Thus, further approaches for optimizing treatment are required. Various biological processes oscillate in a circadian rhythm and are regulated by an endogenous clock. The machinery controlling the circadian cycle is tightly coupled with the cell cycle and can interact with tumor suppressor genes/oncogenes; and can therefore potentially influence cancer progression. Understanding the detailed interactions may lead to the discovery of prognostic and diagnostic biomarkers and new potential targets for treatment. Here, we explain how the circadian system relates to the cell cycle, cancer, and tumor suppressor genes/oncogenes. Furthermore, we propose that circadian clock genes may be potential biomarkers for some cancers and review the current advances in the treatment of PC by targeting the circadian clock. Despite efforts to diagnose pancreatic cancer early, it still remains a cancer with poor prognosis and high mortality rates. While studies have shown the role of molecular clock disruption in tumor initiation, development, and therapy resistance, the role of circadian genes in pancreatic cancer pathogenesis is not yet fully understood and further studies are required to better understand the potential of circadian genes as biomarkers and therapeutic targets.


Similar content being viewed by others
Data availability
Not applicable.
References
Kolenda T, Guglas K, Kopczyńska M, Sobocińska J, Teresiak A, Bliźniak R et al (2020) Good or not good: role of miR-18a in cancer biology. Rep Pract Oncol Radiother 25(5):808–819
Cai J, Chen H, Lu M, Zhang Y, Lu B, You L et al (2021) Advances in the epidemiology of pancreatic cancer: trends, risk factors, screening, and prognosis. Cancer Lett 520:1–11
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Can Res 74(11):2913–2921
Chung V, Sun V, Ruel N, Smith TJ, Ferrell BR (2022) Improving palliative care and quality of life in pancreatic cancer patients. J Palliat Med 25(5):720–727
Tavano F, Pazienza V, Fontana A, Burbaci FP, Panebianco C, Saracino C et al (2015) SIRT1 and circadian gene expression in pancreatic ductal adenocarcinoma: effect of starvation. Chronobiol Int 32(4):497–512
Relles D, Sendecki J, Chipitsyna G, Hyslop T, Yeo CJ, Arafat HA (2013) Circadian gene expression and clinicopathologic correlates in pancreatic cancer. J Gastrointest Surg 17(3):443–450
Hu J-X, Zhao C-F, Chen W-B, Liu Q-C, Li Q-W, Lin Y-Y et al (2021) Pancreatic cancer: a review of epidemiology, trend, and risk factors. World J Gastroenterol 27(27):4298
Daoud AZ, Mulholland EJ, Cole G, McCarthy HO (2019) MicroRNAs in pancreatic cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer 19(1):1130
García-Costela M, Escudero-Feliú J, Puentes-Pardo JD, San Juán SM, Morales-Santana S, Ríos-Arrabal S et al (2020) Circadian genes as therapeutic targets in pancreatic cancer. Front Endocrinol 11:638
Crnko S, Du Pré BC, Sluijter JP, Van Laake LW (2019) Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 16(7):437–447
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549
Yagita K, Horie K, Koinuma S, Nakamura W, Yamanaka I, Urasaki A et al (2010) Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc Natl Acad Sci 107(8):3846–3851
Dierickx P, Vermunt MW, Muraro MJ, Creyghton MP, Doevendans PA, van Oudenaarden A et al (2017) Circadian networks in human embryonic stem cell-derived cardiomyocytes. EMBO Rep 18(7):1199–1212
Stokkan K-A, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291(5503):490–493
Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM et al (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289(5488):2344–2347
Hernández-Rosas F, López-Rosas CA, Saavedra-Vélez MV (2020) Disruption of the molecular circadian clock and cancer: an epigenetic link. Biochem Genet 58(1):189–209
Li Y, Basti A, Yalçin M, Relógio A (2020) Circadian dysregulation of the TGFβ/SMAD4 pathway modulates metastatic properties and cell fate decisions in pancreatic cancer cells. Iscience 23(10):101551
Garrido ALF, de Sousa DA, Santana PT, Rodrigues GH, Pellegrino P, Nogueira LFR et al (2021) Eating habits, sleep, and a proxy for circadian disruption are correlated with dyslipidemia in overweight night workers. Nutrition 83:111084
Morris CJ, Yang JN, Scheer FA (2012) The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res 199:337–358
Morales-Santana S, Morell S, Leon J, Carazo-Gallego A, Jimenez-Lopez JC, Morell M (2019) An overview of the polymorphisms of circadian genes associated with endocrine cancer. Front Endocrinol 10:104
Cash E, Sephton S, Chagpar A, Spiegel D, Rebholz W, Zimmaro L et al (2015) Circadian disruption and biomarkers of tumor progression in breast cancer patients awaiting surgery. Brain Behav Immun 48:102–114
Papantoniou K, Devore EE, Massa J, Strohmaier S, Vetter C, Yang L et al (2018) Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int J Cancer 143(11):2709–2717
Kogevinas M, Espinosa A, Castelló A, Gómez-Acebo I, Guevara M, Martin V et al (2018) Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain study). Int J Cancer 143(10):2380–2389
Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B et al (2012) Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer 131(11):2684–2692
Yang MY, Chang JG, Lin PM, Tang KP, Chen YH, Lin HYH et al (2006) Downregulation of circadian clock genes in chronic myeloid leukemia: alternative methylation pattern of hPER3. Cancer Sci 97(12):1298–1307
Yang X, Wood PA, Oh E-Y, Du-Quiton J, Ansell CM, Hrushesky WJ (2009) Down regulation of circadian clock gene period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res Treat 117(2):423–431
Tokunaga H, Takebayashi Y, Utsunomiya H, Akahira J-I, Higashimoto M, Mashiko M et al (2008) Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand 87(10):1060–1070
Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, Ostrander EA et al (2009) Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study circadian genes and prostate cancer. Can Res 69(24):9315–9322
Pogue-Geile KL, Lyons-Weiler J, Whitcomb DC (2006) Molecular overlap of fly circadian rhythms and human pancreatic cancer. Cancer Lett 243(1):55–57
Udoh US, Valcin JA, Gamble KL, Bailey SM (2015) The molecular circadian clock and alcohol-induced liver injury. Biomolecules 5(4):2504–2537
Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U (2004) The mammalian circadian timing system: from gene expression to physiology. Chromosoma 113:103–112
Kato Y, Kawamoto T, Fujimoto K, Noshiro M (2014) DEC1/STRA13/SHARP2 and DEC2/SHARP1 coordinate physiological processes, including circadian rhythms in response to environmental stimuli. Curr Top Dev Biol 110:339–372
Goriki A, Hatanaka F, Myung J, Kim JK, Yoritaka T, Tanoue S et al (2014) A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol 12(4):e1001839
Yang Y, Xu T, Zhang Y, Qin X (2017) Molecular basis for the regulation of the circadian clock kinases CK1δ and CK1ε. Cell Signal 31:58–65
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M et al (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109(3):307–320
Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci 111(45):16219–16224
Farshadi E, van Der Horst GT, Chaves I (2020) Molecular links between the circadian clock and the cell cycle. J Mol Biol 432(12):3515–3524
Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB et al (2001) Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol 158(5):1793–1801
Bieler J, Cannavo R, Gustafson K, Gobet C, Gatfield D, Naef F (2014) Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Mol Syst Biol 10(7):739
Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302(5643):255–259
Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T et al (2013) NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci 110(5):1592–1599
Laranjeiro R, Tamai TK, Peyric E, Krusche P, Ott S, Whitmore D (2013) Cyclin-dependent kinase inhibitor p20 controls circadian cell-cycle timing. Proc Natl Acad Sci 110(17):6835–6840
Farshadi E, Yan J, Leclere P, Goldbeter A, Chaves I, van der Horst GT (2019) The positive circadian regulators CLOCK and BMAL1 control G2/M cell cycle transition through cyclin B1. Cell Cycle 18(1):16–33
Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Horst GT (2008) Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol 18(4):286–291
Papp SJ, Huber A-L, Jordan SD, Kriebs A, Nguyen M, Moresco JJ et al (2015) DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. Elife 4:e04883
Unsal-Kaçmaz K, Mullen TE, Kaufmann WK, Sancar A (2005) Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol 25(8):3109–3116
Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP (2006) The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22(3):375–382
Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ (2011) An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice (1432–0428 (Electronic)). Diabetologia 54(1):120–124
Chan K, Wong FS, Pearson JA (2022) Circadian rhythms and pancreas physiology: a review. Front Endocrinol 13:920261
Vieira E, Burris TP, Quesada I (2014) Clock genes, pancreatic function, and diabetes. Trends Mol Med 20(12):685–693
Sato T, Sassone-Corsi P (2022) Nutrition, metabolism, and epigenetics: pathways of circadian reprogramming. EMBO Rep 23(5):e52412
Zhao L, Hutchison AT, Heilbronn LK (2021) Carbohydrate intake and circadian synchronicity in the regulation of glucose homeostasis. Curr Opin Clin Nutr Metab Care 24(4):342–348
Zhang C, Tait C, Minacapelli CD, Bhurwal A, Gupta K, Amin R et al (2022) The role of race, sex, and age in circadian disruption and metabolic disorders. Gastro Hep Advances 1(3):471–479
Eastman CI, Tomaka VA, Crowley SJ (2016) Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci Rep 6(1):1–11
Li J, Somers VK, Lopez-Jimenez F, Di J, Covassin N (2021) Demographic characteristics associated with circadian rest-activity rhythm patterns: a cross-sectional study. Int J Behav Nutr Phys Act 18(1):107
Ijaz S, Verbeek J, Seidler A, Lindbohm M-L, Ojajärvi A, Orsini N et al (2013) Night-shift work and breast cancer—a systematic review and meta-analysis. Scand J Work Environ Health 39:431–447
Filipski E, Delaunay F, King VM, Wu M-W, Claustrat B, Gréchez-Cassiau A et al (2004) Effects of chronic jet lag on tumor progression in mice. Can Res 64(21):7879–7885
Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A et al (2016) Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab 24(2):324–331
Sancar A, Van Gelder RN (2021) Clocks, cancer, and chronochemotherapy. Science. https://doi.org/10.1126/science.abb0738
Cash E, Sephton S, Woolley C, Elbehi AM, Anu RI, Ekine-Afolabi B et al (2021) The role of the circadian clock in cancer hallmark acquisition and immune-based cancer therapeutics. J Exp Clin Cancer Res 40(1):1–14
Ye Y, Xiang Y, Ozguc FM, Kim Y, Liu C-J, Park PK et al (2018) The genomic landscape and pharmacogenomic interactions of clock genes in cancer chronotherapy. Cell Syst 6(3):314–328
Pogue-Geile KL, Mackey JA, George RD, Wood PG, Lee KK, Moser AJ et al (2004) A new microarray, enriched in pancreas and pancreatic cancer cDNAs to identify genes relevant to pancreatic cancer. Cancer Genomics Proteomics 1(5–6):371–386
Fu L, Pelicano H, Liu J, Huang P, Lee CC (2002) The circadian gene Period 2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111(1):41–50
Wood PA, Yang X, Taber A, Oh E-Y, Ansell C, Ayers SE et al (2008) Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol Cancer Res 6(11):1786–1793
Lee S, Donehower LA, Herron AJ, Moore DD, Fu L (2010) Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE 5(6):e10995
Oda A, Katayose Y, Yabuuchi S, Yamamoto K, Mizuma M, Shirasou S et al (2009) Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res 29(4):1201–1209
Zhou L, Yu Y, Sun S, Zhang T, Wang M (2018) Cry 1 regulates the clock gene network and promotes proliferation and migration via the Akt/P53/P21 pathway in human osteosarcoma cells. J Cancer 9(14):2480
Cotterchio M, Lowcock E, Bider-Canfield Z, Lemire M, Greenwood C, Gallinger S et al (2015) Association between variants in atopy-related immunologic candidate genes and pancreatic cancer risk. PLoS ONE 10(5):e0125273
Reszka E, Zienolddiny S (2018) Epigenetic basis of circadian rhythm disruption in cancer. Cancer Epigenet Precision Med. https://doi.org/10.1007/978-1-4939-8751-1_10
Bönsch D, Hothorn T, Krieglstein C, Koch M, Nehmer C, Lenz B et al (2007) Daily variations of homocysteine concentration may influence methylation of DNA in normal healthy individuals. Chronobiol Int 24(2):315–326
Xia L, Ma S, Zhang Y, Wang T, Zhou M, Wang Z et al (2015) Daily variation in global and local DNA methylation in mouse livers. PLoS ONE 10(2):e0118101
Doi M, Hirayama J, Sassone-Corsi P (2006) Circadian regulator CLOCK is a histone acetyltransferase. Cell 125(3):497–508
Katada S, Imhof A, Sassone-Corsi P (2012) Connecting threads: epigenetics and metabolism. Cell 148(1–2):24–28
Reszka E, Zienolddiny S (2018) Epigenetic basis of circadian rhythm disruption in cancer. In: Dumitrescu RG, Verma M (eds) Cancer epigenetics for precision medicine: methods and protocols. Springer, New York, pp 173–201
Mao Y, Fu A, Hoffman AE, Jacobs DI, Jin M, Chen K et al (2015) The circadian gene CRY2 is associated with breast cancer aggressiveness possibly via epigenomic modifications. Tumor Biology 36:3533–3539
Alexander M, Burch J, Steck S, Chen C-F, Hurley T, Cavicchia P et al (2017) Case-control study of candidate gene methylation and adenomatous polyp formation. Int J Colorectal Dis 32:183–192
Tomita T, Kurita R, Onishi Y (2017) Epigenetic regulation of the circadian clock: role of 5-aza-2′-deoxycytidine. Biosci Rep. https://doi.org/10.1042/BSR20170053
Hsu M-C, Huang C-C, Choo K-B, Huang C-J (2007) Uncoupling of promoter methylation and expression of Period1 in cervical cancer cells. Biochem Biophys Res Commun 360(1):257–262
Salavaty A, Mohammadi N, Shahmoradi M, Naderi SM (2017) Bioinformatic analysis of circadian expression of oncogenes and tumor suppressor genes. Bioinform Biol Insights 11:1177932217746991
Burchett JB, Knudsen-Clark AM, Altman BJ (2021) MYC Ran up the clock: the complex interplay between MYC and the molecular circadian clock in cancer. Int J Mol Sci 22(14):7761
Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122(5):803–815
Miki T, Matsumoto T, Zhao Z, Lee CC (2013) p53 regulates Period2 expression and the circadian clock. Nat Commun 4(1):1–11
Zou X, Kim DW, Gotoh T, Liu J, Kim JK, Finkielstein CV (2020) A systems biology approach identifies hidden regulatory connections between the circadian and cell-cycle checkpoints. Front Physiol 11:327
Gotoh T, Kim JK, Liu J, Vila-Caballer M, Stauffer PE, Tyson JJ et al (2016) Model-driven experimental approach reveals the complex regulatory distribution of p53 by the circadian factor Period 2. Proc Natl Acad Sci 113(47):13516–13521
Filipski E, Li XM, Lévi F (2006) Disruption of circadian coordination and malignant growth. Cancer Causes Control 17(4):509–514
Mullenders J, Fabius AW, Madiredjo M, Bernards R, Beijersbergen RL (2009) A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS ONE 4(3):e4798
Jiang W, Zhao S, Jiang X, Zhang E, Hu G, Hu B et al (2016) The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett 371(2):314–325
Basti A, Fior R, Yalҫin M, Póvoa V, Astaburuaga R, Li Y et al (2020) The Core-Clock gene NR1D1 impacts cell motility in vitro and invasiveness in a zebrafish xenograft colon cancer model. Cancers 12(4):853
Lee Y, Lahens NF, Zhang S, Bedont J, Field JM, Sehgal A (2019) G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol 17(4):e3000228
Wu Y, Sato F, Yamada T, Bhawal UK, Kawamoto T, Fujimoto K et al (2012) The BHLH transcription factor DEC1 plays an important role in the epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol 41(4):1337–1346
Wang W, Reiser-Erkan C, Michalski CW, Raggi MC, Quan L, Yupei Z et al (2010) Hypoxia inducible BHLHB2 is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun 401(3):422–428
Li W, Liu L, Liu D, Jin S, Yang Y, Tang W et al (2016) Decreased circadian component Bmal1 predicts tumor progression and poor prognosis in human pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun 472(1):156–162
Zeng ZL, Luo HY, Yang J, Wu WJ, Chen DL, Huang P et al (2014) Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin Cancer Res 20(4):1042–1052
Zhang L-L, He Q-K, Lv Y-N, Zhang Z-J, Xiang Y-K (2021) Expression pattern and prognostic value of circadian clock genes in pancreatic adenocarcinoma. Chronobiol Int 38(5):681–693
Xu Z, Wang Z, Jia X, Wang L, Chen Z, Wang S et al (2016) MMGZ01, an anti-DLL4 monoclonal antibody, promotes nonfunctional vessels and inhibits breast tumor growth. Cancer Lett 372(1):118–127
Sato F, Nagata C, Liu Y, Suzuki T, Kondo J, Morohashi S et al (2009) PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem 146(6):833–838
Qiu M-J, Liu L-P, Jin S, Fang X-F, He X-X, Xiong Z-F et al (2019) Research on circadian clock genes in common abdominal malignant tumors. Chronobiol Int 36(7):906–918
Chang WH, Lai AG (2019) Timing gone awry: distinct tumour suppressive and oncogenic roles of the circadian clock and crosstalk with hypoxia signalling in diverse malignancies. J Transl Med 17(1):1–16
Shen GQ, Aleassa EM, Walsh RM, Morris-Stiff G (2019) Next-generation sequencing in pancreatic cancer. Pancreas 48(6):739–748
Miller AL, Garcia PL, Yoon KJ (2020) Developing effective combination therapy for pancreatic cancer: an overview. Pharmacol Res 155:104740
Jiang W, Zhao S, Shen J, Guo L, Sun Y, Zhu Y et al (2018) The MiR-135b–BMAL1–YY1 loop disturbs pancreatic clockwork to promote tumourigenesis and chemoresistance. Cell Death Dis 9(2):1–15
Li S, Hong H, Lv H, Wu G, Wang Z (2016) SIRT 1 overexpression is associated with metastasis of pancreatic ductal adenocarcinoma (PDAC) and promotes migration and growth of PDAC cells. Med Sci Monit 22:1593
Xu J, Zhu W, Xu W, Yao W, Zhang B, Xu Y et al (2013) Up-regulation of MBD1 promotes pancreatic cancer cell epithelial-mesenchymal transition and invasion by epigenetic down-regulation of E-cadherin. Curr Mol Med 13(3):387–400
McGlynn LM, McCluney S, Jamieson NB, Thomson J, MacDonald AI, Oien K et al (2015) SIRT3 & SIRT7: potential novel biomarkers for determining outcome in pancreatic cancer patients. PLoS ONE 10(6):e0131344
Stenzinger A, Endris V, Klauschen F, Sinn B, Lorenz K, Warth A et al (2013) High SIRT1 expression is a negative prognosticator in pancreatic ductal adenocarcinoma. BMC Cancer 13(1):1–12
Gong D-J, Zhang J-M, Yu M, Zhuang B, Guo Q-Q (2013) Inhibition of SIRT1 combined with gemcitabine therapy for pancreatic carcinoma. Clin Interv Aging 8:889–897
Zhang JG, Hong DF, Zhang CW, Sun XD, Wang ZF, Shi Y et al (2014) Sirtuin 1 facilitates chemoresistance of pancreatic cancer cells by regulating adaptive response to chemotherapy-induced stress. Cancer Sci 105(4):445–454
Oon CE, Strell C, Yeong KY, Östman A, Prakash J (2015) SIRT1 inhibition in pancreatic cancer models: contrasting effects in vitro and in vivo. Eur J Pharmacol 757:59–67
Azmi AS, Philip PA, Aboukameel A, Wang Z, Banerjee S, Zafar SF et al (2010) Reactivation of p53 by novel MDM2 inhibitors: implications for pancreatic cancer therapy. Curr Cancer Drug Targets 10(3):319–331
Lanzino M, Maris P, Sirianni R, Barone I, Casaburi I, Chimento A et al (2013) DAX-1, as an androgen-target gene, inhibits aromatase expression: a novel mechanism blocking estrogen-dependent breast cancer cell proliferation. Cell Death Dis 4(7):e724
Lian H, Su M, Zhu Y, Zhou Y, Soomro SH, Fu H (2019) Protein kinase CK2, a potential therapeutic target in carcinoma management. Asian Pac J Cancer Prev 20(1):23–32
Behrend L, Milne DM, Stöter M, Deppert W, Campbell LE, Meek DW et al (2000) IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene 19(47):5303–5313
Battaglin F, Chan P, Pan Y, Soni S, Qu M, Spiller ER et al (2021) Clocking cancer: the circadian clock as a target in cancer therapy. Oncogene 40(18):3187–3200
Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z et al (2019) Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov 9(11):1556–1573
Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A et al (2018) Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553(7688):351–355
Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z et al (2019) Targeting glioblastoma stem cells through disruption of the circadian clock targeting the circadian clock in glioblastoma stem cells. Cancer Discov 9(11):1556–1573
Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL et al (2007) Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci 104(9):3342–3347
Matsunaga N, Kohno Y, Kakimoto K, Hayashi A, Koyanagi S, Ohdo S (2011) Influence of CLOCK on cytotoxicity induced by diethylnitrosamine in mouse primary hepatocytes. Toxicology 280(3):144–151
Yalçin M, El-Athman R, Ouk K, Priller J, Relógio A (2020) Analysis of the circadian regulation of cancer hallmarks by a cross-platform study of colorectal cancer time-series data reveals an association with genes involved in Huntington’s disease. Cancers 12(4):963
Gréchez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F (2008) The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem 283(8):4535–4542
Wang J, Li S, Li X, Li B, Li Y, Xia K et al (2019) Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up-regulating matrix metalloproteinase9 expression. Cancer Cell Int 19(1):1–12
Liu Z, Selby CP, Yang Y, Lindsey-Boltz LA, Cao X, Eynullazada K et al (2020) Circadian regulation of c-MYC in mice. Proc Natl Acad Sci 117(35):21609–21617
Guo F, Tang Q, Chen G, Sun J, Zhu J, Jia Y et al (2020) Aberrant expression and subcellular localization of PER2 promote the progression of Oral squamous cell carcinoma. BioMed Res Int 2020:1–10
Wang Z, Li L, Wang Y (2016) Effects of Per2 overexpression on growth inhibition and metastasis, and on MTA1, nm23-H1 and the autophagy-associated PI3K/PKB signaling pathway in nude mice xenograft models of ovarian cancer. Mol Med Rep 13(6):4561–4568
Mteyrek A, Filipski E, Guettier C, Okyar A, Lévi F (2016) Clock gene Per2 as a controller of liver carcinogenesis. Oncotarget 7(52):85832
Lee CC (2006) Tumor suppression by the mammalian Period genes. Cancer Causes Control 17(4):525–530
Hoffman AE, Zheng T, Ba Y, Stevens RG, Yi C-H, Leaderer D et al (2010) Phenotypic effects of the circadian gene Cryptochrome 2 on cancer-related pathways. BMC Cancer 10(1):1–7
Zheng X, Wu K, Liao S, Pan Y, Sun Y, Chen X et al (2018) MicroRNA-transcription factor network analysis reveals miRNAs cooperatively suppress RORA in oral squamous cell carcinoma. Oncogenesis 7(10):1–18
Harding HP, Lazar MA (1995) The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol 15(9):4791–4802
Acknowledgements
Not applicable.
Funding
This study was supported by a Grant from Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
GP, AMA, MA, and RP performed the data collection. The draft was prepared by GP, DK, HG, HF, and MN. The study was designed and supervised by GP, SMH, GAF, MK, and AA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pourali, G., Ahmadzade, A.M., Arastonejad, M. et al. The circadian clock as a potential biomarker and therapeutic target in pancreatic cancer. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04790-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04790-4