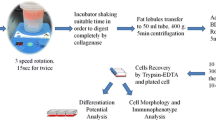
Abstract
The adipose-derived stem cells (ASCs) are a valuable resource for regenerative medicine and essential materials for research in fat deposition. However, the isolation procedure of ASCs has not been standardized and needs to be harmonized; differences in proliferation and adipogenic differentiation of ASCs obtained from different fat depots have not been well characterized. In the present study, we compared the efficiency of ASCs isolation by enzymatic treatment and explant culture methods and the proliferation ability and adipogenic differentiation potential of ASCs isolated from subcutaneous and visceral fat depots. The explant culture method was simple and with no need for expensive enzymes while the enzymatic treatment method was complex, time consuming and costly. By the explant culture method, a larger number of ASCs were isolated from subcutaneous and visceral fat depots. By contrast, fewer ASCs were obtained by the enzymatic treatment method, especially from visceral adipose. ASCs isolated by the explant culture method performed well in cell proliferation and adipogenic differentiation, though they were slightly lower than those by the enzymatic treatment method. ASCs isolated from visceral depot demonstrated higher proliferation ability and adipogenic differentiation potential. In total, the explant culture method is simpler, more efficient, and lower cost than the enzymatic treatment method for ASCs isolation; compared with visceral adipose, subcutaneous adipose is easier to isolate ASCs; however, the visceral ASCs are superior to subcutaneous ASCs in proliferation and adipogenic differentiation.






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228. https://doi.org/10.1089/107632701300062859
Argentati C, Morena F, Bazzucchi M, Armentano I, Emiliani C, Martino S (2018) Adipose stem cell translational applications: from bench-to-bedside. Int J Mol Sci 19:3475. https://doi.org/10.3390/ijms19113475
Baer PC, Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. https://doi.org/10.1155/2012/812693
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, Hui Y (2019) Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114:108765. https://doi.org/10.1016/j.biopha.2019.108765
Baer PC (2020) Adipose-derived stromal/stem cells. Cells 9:1997. https://doi.org/10.3390/cells9091997
Bukowska J, Szostek-Mioduchowska AZ, Kopcewicz M, Walendzik K, Machcinska S, Gawronska-Kozak B (2021) Adipose-derived stromal/stem cells from large animal models: from basic to applied science. Stem Cell Rev Rep 17:719–738. https://doi.org/10.1007/s12015-020-10049-y
Busser H, De Bruyn C, Urbain F, Najar M, Pieters K, Raicevic G, Meuleman N, Bron D, Lagneaux L (2014) Isolation of adipose-derived stromal cells without enzymatic treatment: expansion, phenotypical, and functional characterization. Stem Cells Dev 23:2390–2400. https://doi.org/10.1089/scd.2014.0071
Palumbo P, Lombardi F, Siragusa G, Cifone MG, Cinque B, Giuliani M (2018) Methods of isolation, characterization and expansion of human adipose-derived stem cells (ascs): an overview. Int J Mol Sci 19:1897. https://doi.org/10.3390/ijms19071897
Fraser J, Wulur I, Alfonso Z, Zhu M, Wheeler E (2007) Differences in stem and progenitor cell yield in different subcutaneous adipose tissue depots. Cytotherapy 9:459–467. https://doi.org/10.1080/14653240701358460
Banyard DA, Salibian AA, Widgerow AD, Evans GR (2015) Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med 19:21–30. https://doi.org/10.1111/jcmm.12425
Sampaio RV, Chiaratti MR, Santos DC, Bressan FF, Sangalli JR, Sa AL, Silva TV, Costa NN, Cordeiro MS, Santos SS, Ambrosio CE, Adona PR, Meirelles FV, Miranda MS, Ohashi OM (2015) Generation of bovine (bos indicus) and buffalo (bubalus bubalis) adipose tissue derived stem cells: isolation, characterization, and multipotentiality. Genet Mol Res 14:53–62. https://doi.org/10.4238/2015.January.15.7
Arnhold S, Elashry MI, Klymiuk MC, Geburek F (2019) Investigation of stemness and multipotency of equine adipose-derived mesenchymal stem cells (ascs) from different fat sources in comparison with lipoma. Stem Cell Res Ther 10:309. https://doi.org/10.1186/s13287-019-1429-0
Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208:64–76. https://doi.org/10.1002/jcp.20636
Francis MP, Sachs PC, Elmore LW, Holt SE (2010) Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 6:11–14. https://doi.org/10.4161/org.6.1.10019
Lengi AJ, Corl BA (2010) Factors influencing the differentiation of bovine preadipocytes in vitro. J Anim Sci 88:1999–2008. https://doi.org/10.2527/jas.2009-2439
Shah FS, Wu X, Dietrich M, Rood J, Gimble JM (2013) A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy 15:979–985. https://doi.org/10.1016/j.jcyt.2013.04.001
Zeng G, Lai K, Li J, Zou Y, Huang H, Liang J, Tang X, Wei J, Zhang P (2013) A rapid and efficient method for primary culture of human adipose-derived stem cells. Organogenesis 9:287–295. https://doi.org/10.4161/org.27153
Pond CM (1992) An evolutionary and functional view of mammalian adipose tissue. Proc Nutr Soc 51:367–377. https://doi.org/10.1079/pns19920050
Baer PC, Koch B, Hickmann E, Schubert R, Cinatl JJ, Hauser IA, Geiger H (2019) Isolation, characterization, differentiation and immunomodulatory capacity of mesenchymal stromal/stem cells from human perirenal adipose tissue. Cells. https://doi.org/10.3390/cells8111346
Ritter A, Friemel A, Roth S, Kreis NN, Hoock SC, Safdar BK, Fischer K, Mollmann C, Solbach C, Louwen F, Yuan J (2019) Subcutaneous and visceral adipose-derived mesenchymal stem cells: commonality and diversity. Cells 8:1288. https://doi.org/10.3390/cells8101288
Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL (2013) Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17:644–656. https://doi.org/10.1016/j.cmet.2013.03.008
Schoettl T, Fischer IP, Ussar S (2018) Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol 221:jeb162958. https://doi.org/10.1242/jeb.162958
Jin L, Tang Q, Hu S, Chen Z, Zhou X, Zeng B, Wang Y, He M, Li Y, Gui L, Shen L, Long K, Ma J, Wang X, Chen Z, Jiang Y, Tang G, Zhu L, Liu F, Zhang B, Huang Z, Li G, Li D, Gladyshev VN, Yin J, Gu Y, Li X, Li M (2021) A pig bodymap transcriptome reveals diverse tissue physiologies and evolutionary dynamics of transcription. Nat Commun 12:3715. https://doi.org/10.1038/s41467-021-23560-8
Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ, van Milligen FJ (2008) Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res 332:415–426. https://doi.org/10.1007/s00441-007-0555-7
Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP (2008) Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60:538–544. https://doi.org/10.1097/SAP.0b013e3181723bbe
Zhu R, Feng X, Wei Y, Guo D, Li J, Liu Q, Jiang J, Shi D, Huang J (2021) LncSAMM50 enhances adipogenic differentiation of buffalo adipocytes with no effect on its host gene. Front Genet 12:626158. https://doi.org/10.3389/fgene.2021.626158
Huang J, Guo D, Zhu R, Feng Y, Li R, Yang X, Shi D (2022) FATP1 exerts variable effects on adipogenic differentiation and proliferation in cells derived from muscle and adipose tissue. Front Vet Sci 9:904879. https://doi.org/10.3389/fvets.2022.904879
Song T, Yang Y, Wei H, Xie X, Lu J, Zeng Q, Peng J, Zhou Y, Jiang S, Peng J (2019) Zfp217 mediates m6a mrna methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res 47:6130–6144. https://doi.org/10.1093/nar/gkz312
Qi R, Han X, Wang J, Qiu X, Wang Q, Yang F (2021) Microrna-489–3p promotes adipogenesis by targeting the postn gene in 3t3-l1 preadipocytes. Life Sci 278:119620. https://doi.org/10.1016/j.lfs.2021.119620
Giovanni DP, Franco S (2013) Obesity as a major risk factor for cancer. J Obes 2013:291546. https://doi.org/10.1155/2013/291546
Talmor A, Dunphy B (2015) Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol 29:498–506. https://doi.org/10.1016/j.bpobgyn.2014.10.014
Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127:1–4. https://doi.org/10.1172/JCI92035
Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP (2021) Obesity and cardiovascular disease: a scientific statement from the american heart association. Circulation 143:e984–e1010. https://doi.org/10.1161/CIR.0000000000000973
Zhang Y, Zhang J, Gong H, Cui L, Zhang W, Ma J, Chen C, Ai H, Xiao S, Huang L, Yang B (2019) Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on gwas data in six pig populations. Meat Sci 150:47–55. https://doi.org/10.1016/j.meatsci.2018.12.008
Huang J, Liu X, Feng X, Zhang M, Qu K, Liu J, Wei X, Huang B, Ma Y (2020) Characterization of different adipose depots in fattened buffalo: histological features and expression profiling of adipocyte markers. Arch Anim Breed 63:61–67. https://doi.org/10.5194/aab-63-61-2020
Luo N, Shu J, Yuan X, Jin Y, Cui H, Zhao G, Wen J (2022) Differential regulation of intramuscular fat and abdominal fat deposition in chickens. BMC Genomics 23:308. https://doi.org/10.1186/s12864-022-08538-0
Huang J, Feng X, Zhu R, Guo D, Wei Y, Cao X, Ma Y, Shi D (2020) Comparative transcriptome analysis reveals that pck1 is a potential gene affecting imf deposition in buffalo. BMC Genomics 21:710. https://doi.org/10.1186/s12864-020-07120-w
Wang S, Pan C, Ma X, Yang C, Tang L, Huang J, Wei X, Li H, Ma Y (2021) Identification and functional verification reveals that mir-195 inhibiting thrsp to affect fat deposition in xinyang buffalo. Front Genet 12:736441. https://doi.org/10.3389/fgene.2021.736441
Yamada T, Kamiya M, Higuchi M (2022) Metabolomic analysis of plasma and intramuscular adipose tissue between wagyu and holstein cattle. J Vet Med Sci 84:186–192. https://doi.org/10.1292/jvms.21-0562
Huang J, Zheng Q, Wang S, Wei X, Li F, Ma Y (2019) High-throughput rna sequencing reveals ndufc2-as lncrna promotes adipogenic differentiation in Chinese buffalo (Bubalus bubalis l). Genes (BASEL) 10:689. https://doi.org/10.3390/genes10090689
Hirai S, Matsumoto H, Hino N, Kawachi H, Matsui T, Yano H (2007) Myostatin inhibits differentiation of bovine preadipocyte. Domest Anim Endocrinol 32:1–14. https://doi.org/10.1016/j.domaniend.2005.12.001
Deng Y, Huang G, Chen F, Testroet ED, Li H, Li H, Nong T, Yang X, Cui J, Shi D, Yang S (2019) Hypoxia enhances buffalo adipose-derived mesenchymal stem cells proliferation, stemness, and reprogramming into induced pluripotent stem cells. J Cell Physiol 234:17254–17268. https://doi.org/10.1002/jcp.28342
Ferjak EN, Cavinder CA, Sukumaran AT, Burnett DD, Lemley CO, Dinh T (2019) Fatty acid composition of mesenteric, cardiac, abdominal, intermuscular, and subcutaneous adipose tissues from horses of three body condition scores. Livest Sci 223:116–123. https://doi.org/10.1016/j.livsci.2019.02.010
Costa AS, Lopes PA, Estevao M, Martins SV, Alves SP, Pinto R, Pissarra H, Correia JJ, Pinho M, Fontes C, Prates J (2012) Contrasting cellularity and fatty acid composition in fat depots from alentejana and barrosa bovine breeds fed high and low forage diets. Int J Biol Sci 8:214–227. https://doi.org/10.7150/ijbs.8.214
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156:20–44. https://doi.org/10.1016/j.cell.2013.12.012
Sobczuk-Szul M, Mochol M, Nogalski Z, Pogorzelska-Przybylek P (2021) Fatty acid profile as affected by fat depot and the sex category of polish holstein-friesian x limousin fattening cattle fed silage ad libitum. Anim Sci J 92:e13516. https://doi.org/10.1111/asj.13516
Acknowledgements
We would like to thank the Guangxi Bagui Scholar Program, Innovation Project of Guangxi Graduate Education and Bama County Program for Talents in Science and Technology.
Funding
This study was supported by the National Natural Science Foundation of China (32060747 and U20A2051) and the Guangxi Natural Science Foundation (2020JJA130143 and 2021AC19014).
Author information
Authors and Affiliations
Contributions
JH, YM, and DS: concepts, design. RZ, YF, RL and KW, QL: material preparation. RZ, YF: experimental studies. RZ: validation, data analysis, software, and validation. JH: writing—reviewing and editing, funding acquisition, and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All animal protocols were approved by the Animal Care Committee of the College of Animal Science and Technology, Guangxi University (Gxu-2021-050).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, R., Feng, Y., Li, R. et al. Isolation methods, proliferation, and adipogenic differentiation of adipose-derived stem cells from different fat depots in bovines. Mol Cell Biochem 479, 643–652 (2024). https://doi.org/10.1007/s11010-023-04753-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04753-9