Abstract
Fibrodysplasia Ossificans Progressiva (FOP) is a rare genetic disease caused by heterozygous missense mutations in Activin A receptor type I which is also known as Activin-like kinase 2 (ALK2), a type I receptor of Bone Morphogenetic Proteins(BMP). Patients with FOP usually undergo episodic flare-ups and the heterotopic ossification in soft and connective tissues. Molecular mechanism study indicates that Activin A, the ligand which normally transduces Transforming Growth Factor Beta signaling, abnormally activates BMP signaling through ALK2 mutants in FOP, leading to heterotopic bone formation. To date, effective therapies to FOP are unavailable. However, significant advances have recently been made in the development of FOP drugs. In this article, we review the recent advances in understanding the FOP mechanism and drug development, with a focus on the small-molecular and antibody drugs currently in the clinical trials for FOP treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
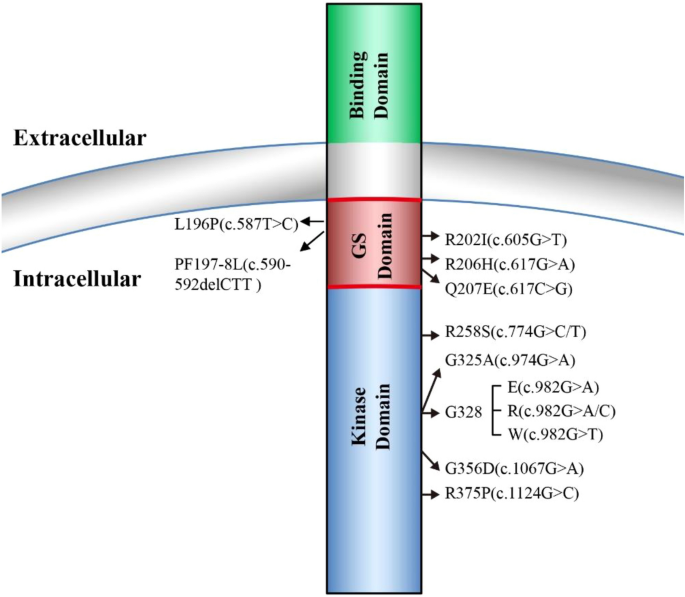
FOP is a rare human genetic disorder in which ectopic bone formation occurs in connective tissue such as tendons, ligaments, and skeletal muscles throughout the body, leading to progressive loss of mobility, chronic pain, and eventual premature death mainly due to cardiorespiratory failure [1]. A worldwide prevalence of FOP is approximately one in two million population without ethnic, racial, or geographic predisposition [2]. One main symptom of FOP is a malformation of big toes at birth which also serves as an early diagnostic hallmark for FOP [2, 3]. In 2006, the first heterozygous missense causative mutation of FOP (617G>A; R206H) was reported in the gene-encoding ACVR1 [4]. Since then, additional new heterozygous missense causative mutations in ACVR1 have been reported, and further studies indicated that ACVR1R206H mutation occurs in approximately 97% of FOP patients [5, 6] (Fig. 1). ACVR1, also known as ALK2, is a type I receptor of BMP signaling essential for normal skeleton formation and embryonic patterning [7, 8]. For a more complete view of FOP etiology, clinical characteristics, diagnosis, and management, we refer the readers to the excellent reviews in these topics [2, 3, 9].
Early mechanistic studies showed that FOP ALK2 mutants result in leaky BMP signaling in a basal condition and hyper-responsiveness upon BMP ligand stimulation [10,11,12,13,14,15,16,17]. However, recent findings have confirmed that activin A, the ligand which normally transduces TGF-β signaling, abnormally activates BMP signaling through FOP-mutated ALK2 [18,19,20,21]. This abnormal activin A-induced BMP signaling is thought to trigger heterotopic ossification of connective tissues [22]. To date, although effective therapies for FOP are unavailable, significant advances have been achieved in the development of potential FOP drugs, resulting in several promising therapies currently in clinical trials [23]. In this article, we review the recent progress in FOP mechanism studies and drug development, with a focus on the small-molecular and antibody drugs in the clinical trials for FOP treatment.
BMP signaling and FOP
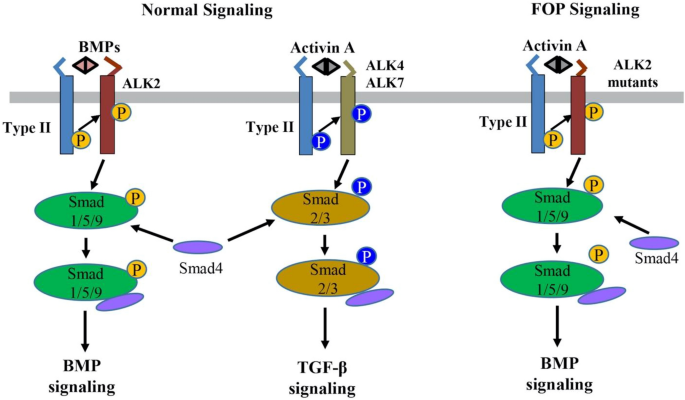
BMPs are secreted multi-functional growth factors, and they belong to the TGF-β super family. BMPs consist of more than 20 family members which play central roles in regulating cellular morphogenesis, differentiation, proliferation, and apoptosis during embryogenesis and adult homeostasis [24]. The BMPs signal transduction is mainly mediated through the canonic Smads-dependent pathway in which BMPs first bind to a heterotetrametric complex consisting of a type II receptor homodimer and a type I receptor homodimer (Fig. 2). Then the type II receptors phosphorylate and activate the type I receptors, which in turn phosphorate Smad1/5/9 (also known as Smad1/5/8). The phosphorylated Smad1/5/9 subsequently form a complex with Smad4, which then translocates into the nucleus where it binds to BMP response elements and activates transcription of BMPs target genes [24, 25].
The normal BMP/TGF-β signaling pathways and abnormal activin A-induced BMP signaling through the ALK2 mutants in FOP. BMP or activin A ligands assemble and bind to a heterotetramer complex consisting of a type II receptor homodimer and a type I receptor homodimer (e.g., ALK2 for BMP and ALK4/7 for activin A). The type II receptor phosphorylates the type I receptor, which subsequently phosphorylates Smads (Smad1/5/9 for BMPs and Smad2/3 for activin A) to transduce normal BMP and TGF-β signaling, respectively. In contrast, in FOP, activin A can abnormally cross-signal BMP signaling through the ALK2 mutants
Four type I receptors, ALK1, ALK2, ALK3, and ALK6, are able to mediate BMP signaling and malfunctions of these four types I receptors are involved in many diseases including cancer [26, 27]. In FOP, the most common mutation R206H is located at the intracellular glycine-serine-rich (GS) domain of ALK2, where FKBP12 protein (also known as FKBP1A) binds to ALK2 to prevent ALK2 activation in the absence of BMP ligands [12, 15, 16]. ALK2R206H has been shown to induce basal leaky BMP signaling in the absence of BMP ligands and hyper-responsiveness upon BMP ligand stimulation that was initially thought to result in the ectopic endochondral ossification in FOP [15,16,17, 28]. Later, additional FOP mutations have been identified in both GS domain and kinase domain of ALK2, which are associated with the disease onset ages and the extent of heterotopic ossification [5, 10, 29,30,31,32].
Nevertheless, recent findings have proved that activin A, a ligand which normally transduces TGF-β signaling, abnormally activates BMP signaling in FOP [18,19,20,21]. In normal physiological conditions, BMPs utilize ALK1/ALK2/ALK3/ALK6 as the type I receptors to activate Smad1/5/9-dependent BMP signaling, while activin A signals through ALK4/ALK7 as the type I receptors for Smad2/3-dependent TGF-β signaling and activin A does not transduce Smad1/5/9-dependent BMP signaling [33] (Fig. 2). However, recent multiple studies have demonstrated that activin A can activate Smad1/5/9-dependent BMP signaling in cells expressing ALK2R206H in vitro and induced heterotopic ossification in a conditional knock-in mouse model of FOP in vivo [18,19,20,21, 34, 35]. In addition, this heterotopic ossification in the FOP mouse model can be blocked by the activin A-specific antibodies supporting that activin A cross-signal BMP pathway via mutated FOP ALK2 receptors [18,19,20,21]. Advances in understanding of the FOP molecular mechanism have led to significant progress in FOP drug development.
Recent drug development for FOP
Based on the molecular mechanism underlying FOP, multiple potential therapeutic targets have been selected for drug development to treat the disease.
Targeting ALK2
Since FOP is caused by the missense mutations of ALK2, ALK2 has been long thought as a potential therapeutic target for FOP and significant efforts have been made to develop ALK2 inhibitors.
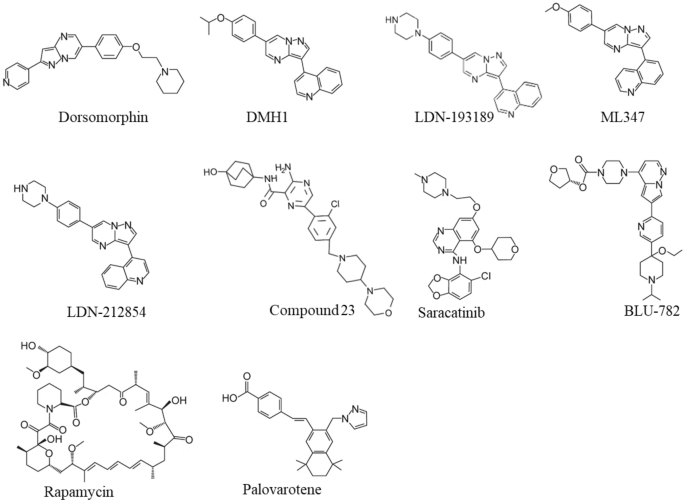
Dorsomorphin, the first ALK2 chemical inhibitor, was identified from an in vivo screening of BMP inhibitors using zebrafish embryos [36] (Fig. 3). Unfortunately, Dorsomorphin displays notable off-targets against serval other kinases including Vascular Endothelial Growth Factor Receptor 2 (VEGFR2), ALK5, AMP-activated kinase (AMPK) and platelet-derived growth factor receptor β (PDGFRβ) [37], raising concerns about its clinical safety [37, 38]. To develop more selective ALK2 inhibitors, we and colleagues have synthesized 63 Dorsomorphin analogs and identified DMH1 from those analogs by using zebrafish embryo screening [37]. In contrast to Dorsomorphin, DMH1 is more selective to ALK2, and it does not exhibit detectable activities against the closely related kinases such as VEGFR2, ALK5, AMPK, and PDGFRβ [37]. Meanwhile, another ALK2 inhibitor, LDN-193189, was developed, and it shows better potency and selectivity than Dorsomorphin [39] (Fig. 3). Nevertheless, both DMH1 and LDN-193189 cannot well distinguish ALK2 from other BMP type I receptors (ALK1/3/6) which are essential for development and homeostasis [40,41,42,43]. Therefore, developing better ALK2 inhibitor is critical for FOP treatment with minimum side effects. Further investigations discovered more selective ALK2 inhibitors, ML347 and LDN-212854 with negligible inhibitory activities for all other kinases except ALK1 [44, 45] (Fig. 3). Very recently, Ullrich et al. reported a new potent and selective ALK2 inhibitor, compound 23, which displays excellent biochemical and cellular potency, selectivity, and a favorable in vitro profiles for absorption, distribution, metabolism, and excretion [46]. However, none of the above selective ALK2 inhibitors have moved into clinical trials.
Recently, Williams et al. screened over 220 small-molecular kinase inhibitors which have either been approved previously by FDA or in clinical trials [47]. They identified a potent and selective ALK2 inhibitor, Saracatinib (also known as AZD0530), an orally bioavailable drug developed by AstraZeneca for the treatment of ovarian adenocarcinoma [47, 48] (Fig. 3). Since Saracatinib effectively blocks heterotopic ossification in preclinical FOP models and displays excellent pharmacokinetic parameters and safety, Phase II clinical trial of Saracatinib for FOP was recently initiated in August 2020 (NCT04307953) [49, 50] (Table 1). Another selective ALK2 inhibitor, INCB000928 that was originally developed to treat anemia as an iron homeostasis modulator, is now being evaluated for the efficacy and tolerability in the treatment of FOP in the phase II clinical trial (NCT05090891) [51, 52] (Table 1). Other than small-molecular ALK2 inhibitors, an anti-ALK2 monoclonal antibody, DS-6016a, was developed as well by Daiichi Sankyo and Saitama Medical University in Japan. The Phase I clinical trial of DS-6016a to assess its safety, tolerability, and pharmacokinetics in healthy participants is ongoing, and the study results have not been released to date (NCT04818398) [53] (Table 1).
Nevertheless, these ALK2-targeting potential drugs indiscriminately target both wild-type ALK2 and FOP-mutated ALK2, leading to inhibition of important physiologic BMP signaling essential for normal cellular and tissue function. To overcome this challenge, Blueprint Medicines, Inc. developed a small molecule called BLU-782 (also known as IPN60130), which selectively targets the FOP-mutated ALK2 with minimal interference to the wild-type ALK2 [54] (Fig. 3). The Phase I clinical trial BLU-782 in healthy volunteers to establish its safety of the investigational drug was recently completed (NCT03858075), and the result showed that BLU-782 is well tolerated with approximately 24 h of half-life and displays excellent properties of pharmacokinetics and pharmacodynamics [55, 56] (Table 1).
Targeting activin A
Activin A normally mediates TGF-β signaling by using Activin Receptors type IIA or IIB (ActR-IIA/ActR-IIB) as type II receptors and ALK4/7 as type I receptors followed by the downstream-phosphorylated Smad2/3 as intracellular signal transducers (Fig. 2). However, recent studies have confirmed that activin A abnormally activates BMP-Smad1/5/9 signaling through mutant ALK2 in FOP [18,19,20,21, 34, 35]. Given this interesting discovery, activin A has become a promising therapeutic target for FOP treatment. REGN2477 (also known as Garetosmab), a human anti-activin A-neutralizing antibody, was examined in the FOP mouse model, and the result showed that REGN2477 effectively inhibited heterotopic ossification [19]. The Phase I clinical trial of REGN2477 was completed, and the result demonstrated that REGN2477 displays great safety, tolerability, and pharmacokinetics [57]. Recently its Phase II clinical trial was initiated with a plan to administer 10 mg/kg REGN2477 intravenously every 4 weeks to FOP patients (NCT03188666) [58]. As activin A also plays important roles in multiple biological functions such as ovarian follicle maturation, spermatogenesis, steroidogenesis, muscle growth, immunity, inflammation, neuronal differentiation, and bone remodeling [59,60,61,62,63,64], the potential side effects of REGN2477 for activin A inhibition must be carefully monitored in FOP patients (Table 1).
Targeting other associated transcriptional effectors
It is believed that activin A induces chondrogenesis via BMP signaling in FOP by differentiating connective tissue progenitor cells into chondrocytes and osteoblasts prior to eventual formation of heterotopic bones in soft tissues [34, 65]. Thus, inhibition of chronogenesis may be a good strategy to prevent heterotopic ossification in FOP.
Rapamycin
Rapamycin (also known as Sirolimus) is an immunosuppressive drug used to prevent transplant rejection and lymphangioleiomyomatosis, and it has been recently identified as a potential drug for the treatment of FOP (Fig. 3). In a high-throughput screening by using FOP patient-derived induced pluripotent stem cells (FOP-iPSCs) to identify signaling pathways involved in activin A-induced chondrogenesis, Hino et al. found that the mammalian target of rapamycin (mTOR) signaling is critical in enhanced chondrogenesis initiated by activin A and heterotopic ossification in FOP [66]. They further showed that Rapamycin attenuated heterotopic ossification in both FOP-ALK2R206H conditional transgenic mice and the mice with activin A-triggered heterotopic ossification derived from FOP-iPSCs [66]. Given the promising preclinical studies and its proved safety profile, Phase II/III clinical trials of Rapamycin for randomized, placebo-controlled studies and subsequent open-label extension studies were initiated at Kyoto University Hospital in Japan (UMIN000028429), and the outcomes of this trial has not been publicly released (Table 1). Nevertheless, a case report recently showed that Rapamycin did not show clear benefits to heterotopic ossification reduction in two young patients with classic FOP-ALK2R206H mutation at the administrated dose [67].
Palovarotene
Retinoid signaling mediated by nuclear retinoic acid receptors (RAR) plays a critical biological role in chondrogenesis and normal skeleton formation and retinoic acid signaling agonists could effectively block chondrogenesis and subsequent heterotopic ossification in FOP [68,69,70,71]. In 2011, Shimono et al. showed that palovarotene (also known as R667), a specific agonist of the retinoic acid signaling by targeting nuclear retinoic acid receptor-γ (RARγ) with well characterized safety profile, inhibited heterotopic ossification in a transgenic mouse model expressing ALK2Q207D mutation [72] (Fig. 3). Later, Chakkalakal et al. examined palovarotene in a knock-in mouse model carrying the classic FOP-ALK2R206H mutation and demonstrated that palovarotene effectively blocks trauma-induced and spontaneous heterotopic ossification without comprising limb mobility and growth [73]. Importantly, palovarotene maintained joint, limb, and body motion, providing clear evidence for its encompassing therapeutic potential as a treatment for FOP [73]. In 2014, Clementia Pharmaceuticals initiated a double-blinded, placebo-controlled Phase II clinical trial to evaluate whether palovarotene prevents heterotopic ossification during and following a flare-up in FOP patients (NCT02190747). The trial was completed in 2016, and the result shows that palovarotene reduces the percentage of FOP patients developing heterotopic ossification, the time to remission and patient-reported pain associated with the flare-up area [74]. Currently, the Phase III clinical trial of palovarotene in FOP patients is in progress (NCT03312634). In addition, the rollover Phase III study was launched in November 2021 to further evaluate the safety and efficacy of palovarotene in adult and pediatric participants with FOP who have previously received palovarotene treatment (NCT05027802) [75] (Table 1).
Conclusion
In recent years, significant progresses have been made in understanding the molecular mechanism underlying FOP and developing FOP therapies. The discovery of causative mutations in ALK2 has made it a promising druggable target for FOP. Numerous small-molecular inhibitors and antibodies targeting ALK2 have been developed. Among them, Saracatinib, DS-6016a, and BLU-782 are currently in FOP clinical trials. In addition, as activin A abnormally transduces BMP signaling in FOP, REGN2477 antibody-targeting activin A has been studied for the treatment of FOP, and its efficacy is currently under evaluation in a Phase II clinical trial. Moreover, potential drugs targeting transcriptional effectors associated with the early heterotopic ossification have also shown promise in the treatment of FOP, and their efficacies are being evaluated in clinical trials. For instance, a Phase II clinical trial has showed that RARγ agonist Palovarotene effectively reduces the percentage of FOP patients developing heterotopic ossification and the time to remission (NCT02190747) [74]. Additionally, Rapamycin was shown to attenuate heterotopic ossification in FOP mouse models [66], and a Phase II clinical trial for Rapamycin is currently ongoing. In summary, rapid, and exciting advances have been made in our understating of FOP mechanism and drug development. Several potential drugs are currently under clinical trials to treat FOP at multiple targets, which allows more effective combinatorial pharmacological management for FOP. Nevertheless, as physiological BMP signaling is critical to homeostasis and indiscriminately blocking BMP signaling to treat FOP may raise some concerns, therapeutic agents like BLU-782 that selectively targets only the mutant ALK2 with minimal interference to the wild-type ALK2 may represent an excellent strategy for FOP treatment in the future.
Data availability
Not applicable.
References
Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T et al (2008) Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP). J Bone Miner Metab 26(6):521–530
Pignolo RJ, Shore EM, Kaplan FS (2013) Fibrodysplasia ossificans progressiva: diagnosis, management, and therapeutic horizons. Pediatr Endocrinol Rev 10(Suppl 2):437–448
Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J et al (2008) Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics 121(5):e1295–e1300
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH et al (2006) A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38(5):525–527
Furuya H, Ikezoe K, Wang L, Ohyagi Y, Motomura K, Fujii N et al (2008) A unique case of fibrodysplasia ossificans progressiva with an ACVR1 mutation, G356D, other than the common mutation (R206H). Am J Med Genet A 146A(4):459–463
Kaplan FS, Groppe JC, Xu M, Towler OW, Grunvald E, Kalunian K et al (2022) An ACVR1(R375P) pathogenic variant in two families with mild fibrodysplasia ossificans progressiva. Am J Med Genet Part A 188(3):806–817
Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massague J (2001) The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell 8(3):671–682
Huse M, Chen YG, Massague J, Kuriyan J (1999) Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 96(3):425–436
Haga N, Nakashima Y, Kitoh H, Kamizono J, Katagiri T, Saijo H et al (2020) Fibrodysplasia ossificans progressiva: review and research activities in Japan. Pediatr Int 62(1):3–13
Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L et al (2009) Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30(3):379–390
Groppe JC, Shore EM, Kaplan FS (2007) Functional Modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat R 462:87–92
Groppe JC, Wu J, Shore EM, Kaplan FS (2011) In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs 194(2–4):291–295
Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT et al (2012) Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem 287(44):36990–36998
Bagarova J, Vonner AJ, Armstrong KA, Borgermann J, Lai CS, Deng DY et al (2013) Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 33(12):2413–2424
Shen Q, Little SC, Xu MQ, Haupt J, Ast C, Katagiri T et al (2009) The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 119(11):3462–3472
Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY et al (2010) Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem 285(29):22542–22553
van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J et al (2010) ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Min Res 25(6):1208–1215
Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M et al (2015) Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci USA 112(50):15438–15443
Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L et al (2015) ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aac4358
Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I et al (2015) Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal 13:27
Upadhyay J, Xie L, Huang L, Das N, Stewart RC, Lyon MC et al (2017) The expansion of heterotopic bone in fibrodysplasia ossificans progressiva is activin A-dependent. J Bone Min Res. https://doi.org/10.1002/jbmr.3235
Alessi Wolken DM, Idone V, Hatsell SJ, Yu PB, Economides AN (2018) The obligatory role of Activin A in the formation of heterotopic bone in fibrodysplasia ossificans progressiva. Bone 109:210–217
Connor JM, Woodrow JC, Evans DAP (1982) Histocompatibility antigens in patients with ectopic ossification due to fibrodysplasia ossificans progressiva. Ann Rheum Dis 41(6):646–647
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23(4):609–620
Sanchez-Duffhues G, Williams E, Goumans MJ, Heldin CH, Ten Dijke P (2020) Bone morphogenetic protein receptors: structure, function and targeting by selective small molecule kinase inhibitors. Bone 138:115472
Loomans HA, Andl CD (2016) Activin receptor-like kinases: a diverse family playing an important role in cancer. Am J Cancer Res 6(11):2431–2447
Lin S, Svoboda KK, Feng JQ, Jiang X (2016) The biological function of type I receptors of bone morphogenetic protein in bone. Bone Res 4:16005
Botello-Smith WM, Alsamarah A, Chatterjee P, Xie C, Lacroix JJ, Hao J et al (2017) Polymodal allosteric regulation of type 1 serine/threonine kinase receptors via a conserved electrostatic lock. PLoS Comput Biol 13(8):e1005711
Whyte MP, Wenkert D, Demertzis JL, DiCarlo EF, Westenberg E, Mumm S (2012) Fibrodysplasia ossificans progressiva: middle-age onset of heterotopic ossification from a unique missense mutation (c.974G>C, p.G325A) in ACVR1. J Bone Min Res 27(3):729–737
Bocciardi R, Bordo D, Di Duca M, Di Rocco M, Ravazzolo R (2009) Mutational analysis of the ACVR1 gene in Italian patients affected with fibrodysplasia ossificans progressiva: confirmations and advancements. Eur J Hum Genet 17(3):311–318
Petrie KA, Lee WH, Bullock AN, Pointon JJ, Smith R, Russell RG et al (2009) Novel mutations in ACVR1 result in atypical features in two fibrodysplasia ossificans progressiva patients. PLoS ONE 4(3):e5005
Cappato S, Traberg R, Gintautiene J, Zara F, Bocciardi RA (2021) case of fibrodysplasia ossificans progressiva associated with a novel variant of the ACVR1 gene. Mol Genet Genomic 9(10):e1774
Massague J (2012) TGF beta signalling in context. Nat Rev Mol Cell Biol 13(10):616–630
Wang H, Shore EM, Pignolo RJ, Kaplan FS (2018) Activin A amplifies dysregulated BMP signaling and induces chondro-osseous differentiation of primary connective tissue progenitor cells in patients with fibrodysplasia ossificans progressiva (FOP). Bone 109:218–224
Xie C, Jiang WJ, Lacroix JJ, Luo Y, Hao JJ (2020) Insight into molecular mechanism for activin A-induced bone morphogenetic protein signaling. Int J Mol Sci 21(18):6498
Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA et al (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4(1):33–41
Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR et al (2010) In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 5(2):245–253
Luo Y, Alsamarah A, Zhang K, Hao J (2016) Development of new therapeutic agents for fibrodysplasia ossificans progressiva. Curr Mol Med 16(1):4–11
Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS et al (2008) Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett 18(15):4388–4392
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21(7):1743–1753
Hu-Lowe DD, Chen EH, Zhang LL, Watson KD, Mancuso P, Lappin P et al (2011) Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Can Res 71(4):1362–1373
Chida A, Shintani M, Nakayama T, Furutani Y, Hayama E, Inai K et al (2012) Missense mutations of the BMPR1B (ALK6) gene in childhood idiopathic pulmonary arterial hypertension. Circ J 76(7):1501–1804
Cunha SI, Pietras K (2011) ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood 117(26):6999–7006
Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR (2013) Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a] pyrimidine scaffold of Dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorganic Med Chem Lett 23(11):3248
Mohedas AH, Xing XC, Armstrong KA, Bullock AN, Cuny GD, Yu PB (2013) Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem Biol 8(6):1291–1302
Ullrich T, Arista L, Weiler S, Teixeira-Fouchard S, Broennimann V, Stiefl N et al (2022) Discovery of a novel 2-aminopyrazine-3-carboxamide as a potent and selective inhibitor of activin receptor-like kinase-2 (ALK2) for the treatment of fibrodysplasia ossificans progressiva. Bioorg Med Chem Lett. https://doi.org/10.1016/j.bmcl.2022.128667
Williams E, Bagarova J, Kerr G, Xia DD, Place ES, Dey D et al (2021) Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva. JCI Insight. https://doi.org/10.1172/jci.insight.95042
Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP et al (2006) N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem 49(22):6465–6488
Connor JM (1996) Fibrodysplasia ossificans progressive: lessons from rare maladies. N Engl J Med 335(8):591–593
Saracatinib Trial TO Prevent FOP (2020). https://clinicaltrials.gov/ct2/show/NCT04307953. Accessed 12 Dec 2021
Chen YY, Stubbs MC, Pusey M, Wen XM, Collins RJ, Kapilashrami K et al (2020) Characterization of INCB00928, a potent and selective ALK2 inhibitor for the treatment of anemia. Blood 136:52–52
To assess the efficacy, safety, and tolerability of INCB000928 in participants With Fibrodysplasia Ossificans Progressiva (2021). https://clinicaltrials.gov/ct2/show/NCT05090891. Accessed 12 Dec 2021
Study of single-ascending doses of DS-6016a in healthy Japanese subjects (2021). https://clinicaltrials.gov/ct2/show/NCT04818398. Accessed 12 Dec 2021
Blueprint medicines presents foundational preclinical data supporting the development of BLU-782, a highly selective ALK2 inhibitor, for the treatment of patients with fibrodysplasia ossificans progressive (2018)
Safety, tolerability, pharmacokinetics, and food effect of BLU-782 in healthy adults (2019). https://clinicaltrials.gov/ct2/show/NCT03858075. Accessed 14 Dec 2021
Alison DFA, Riadh L, Michael P, Cori AS, Sara G, Faith S, Sean K, Gordon W, Mark H, Robert S, Rachel S, Morgan L, Pauplis R, Vivek K, Andy B, Timothy L (2019) A clinical update on BLU-782, an investigational ALK2 inhibitor in development for fibrodysplasia ossificans progressiva (FOP). https://www.blueprintmedicines.com/wp-content/uploads/2019/09/Blueprint-Medicines-ASBMR-2019-BLU-782-Poster1.pdf.
Vanhoutte F, Liang S, Ruddy M, Zhao A, Drewery T, Wang Y et al (2020) Pharmacokinetics and pharmacodynamics of garetosmab (Anti-activin A): results from a first-in-human phase 1 study. J Clin Pharmacol 60(11):1424–1431
A study to examine the safety, tolerability and effects on abnormal bone formation of REGN2477 in patients with fibrodysplasia ossificansprogressiva (2017). https://clinicaltrials.gov/ct2/show/NCT03188666. Accessed 16 Dec 2021
Ries A, Schelch K, Falch D, Pany L, Hoda MA, Grusch M (2020) Activin A: an emerging target for improving cancer treatment? Expert Opin Ther Targets 24(10):985–996
Knight PG, Satchell L, Glister C (2012) Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol 359(1–2):53–65
Hedger MP, Winnall WR (2012) Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol Cell Endocrinol 359(1–2):30–42
Petrakou E, Fotopoulos S, Anagnostakou M, Anatolitou F, Samitas K, Semitekolou M et al (2013) Activin-A exerts a crucial anti-inflammatory role in neonatal infections. Pediatr Res 74(6):675–681
Mayer K, Buchbinder A, Morty RE (2012) Activin A: a mediator governing inflammation, immunity, and repair. Am J Respir Crit Care Med 185(4):350–352
Rodriguez-Martinez G, Molina-Hernandez A, Velasco I (2012) Activin A promotes neuronal differentiation of cerebrocortical neural progenitor cells. PLoS ONE 7(8):e43797
Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA et al (2018) Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun 9(1):471
Hino K, Horigome K, Nishio M, Komura S, Nagata S, Zhao C et al (2017) Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J Clin Invest 127(9):3339–3352
Kaplan FS, Zeitlin L, Dunn SP, Benor S, Hagin D, Al Mukaddam M et al (2018) Acute and chronic rapamycin use in patients with fibrodysplasia ossificans progressiva: a report of two cases. Bone 109:281–284
Pacifici M (2018) Retinoid roles and action in skeletal development and growth provide the rationale for an ongoing heterotopic ossification prevention trial. Bone 109:267–275
Underhill TM, Cash DE, Linney E (1994) Constitutively active retinoid receptors exhibit interfamily and intrafamily promoter specificity. Mol Endocrinol 8(3):274–285
Weston AD, Rosen V, Chandraratna RA, Underhill TM (2000) Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J Cell Biol 148(4):679–690
Weston AD, Hoffman LM, Underhill TM (2003) Revisiting the role of retinoid signaling in skeletal development. Birth Defects Res C Embryo Today 69(2):156–173
Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C et al (2011) Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med 17(4):454–460
Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS et al (2016) palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1(R206H) fibrodysplasia ossificans progressiva (FOP) Mutation. J Bone Min Res 31(9):1666–1675
Kaplan FS, Hsiao EC, Baujat G, Keen R, Grogan DR, Pignolo RJ (2017) Efficacy and safety of palovarotene in fibrodysplasia ossificans progressiva (FOP): a randomized, placebo-controlled, double-blind study. J Bone Min Res 32:S114–S114
An efficacy and safety study of palovarotene for the treatment of fibrodysplasia ossificans progressiva (2017). https://clinicaltrials.gov/ct2/show/NCT03312634. Accessed 15 Dec 2021
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
XM and HW reviewed articles, prepared figures, and wrote original draft. JH supervised the review, organized the figures, wrote, and edited the manuscript. All authors contributed to the critical reading and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a review article, and no ethical approval is required.
Consent for publication
Its publication has been approved by all co-authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meng, X., Wang, H. & Hao, J. Recent progress in drug development for fibrodysplasia ossificans progressiva. Mol Cell Biochem 477, 2327–2334 (2022). https://doi.org/10.1007/s11010-022-04446-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04446-9