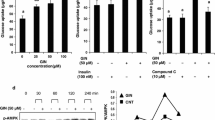
Abstract
Diabetes type 1 (T1D) characterized by destruction of pancreatic β-cells results in inadequate insulin production and hyperglycaemia. Generation of reactive oxygen species and glycosylation end-products stimulates toxic impacts on T1D. Dietary w-3 fatty acids present in Fish oil (FO) might be helpful in the prevention of oxidative stress and lipid peroxidation, thus, beneficial against T1D. But how the cellular secretion from β-cells under influence of FO affects the glucose homeostasis of peri-pancreatic cells is poorly understood. In the current study, we aimed to introduce an in vitro model for T1D and evaluate its effectiveness in respect of alloxan treatment to pancreatic Min6 cells. We use alloxan in the Min6 pancreatic β-cell line to induce cellular damage related to T1D. Further treatment with FO was seen to prevent cell death by alloxan and induce mRNA expression of both insulin 1 and insulin 2 isoforms under low-glucose conditions. From the first part of the study, it is clear that FO is effective to recover Min6 cells from the destructive effect of alloxan, and it worked best when given along with alloxan or given after alloxan treatment regime. FO-induced secretion of molecules from Min6 was clearly shown to regulate mRNA expression of key enzymes of carbohydrate metabolism in peri-pancreatic cell types. This is a pilot study showing that an improved in vitro approach of using Min6 along with muscle cells (C2C12) and adipose tissue cells (3T3-L1) together to understand the crosstalk of molecules could be used to check the efficacy of an anti-diabetic drug.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Homo-Delarche F (2004) Neuroendocrine immuno-ontogeny of the pathogenesis of autoimmune diabetes in the nonobese diabetic (NOD) mouse. ILAR J 45(3):237–258
Argilés JM, López-Soriano J, Ortiz MA, Pou JM, López-Soriano FJ (1992) Bimodal effect of transforming growth factor-β on insulin secretion in MIN6 cells. Endocr Rev 13(3):515–524
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383(9911):69–82
Janež A, Guja C, Mitrakou A, Lalic N, Tankova T, Czupryniak L, Tabák AG, Prazny M, Martinka E, Smircic-Duvnjak L (2020) Insulin therapy in adults with type 1 diabetes mellitus: a narrative review. Diabetes Ther: Res Treat Educ Diabetes Relat Disord 11(2):387–409
Kottaisamy CPD, Raj DS, Prasanth Kumar V et al (2021) Experimental animal models for diabetes and its related complications—a review. Lab Anim Res 37:23
Al-Awar A, Kupai K, Veszelka M, Szűcs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga C (2016) Experimental diabetes mellitus in different animal models. J Diabetes Res 2016:9051426. https://doi.org/10.1155/2016/9051426
Kaufman-Francis K, Koffler J, Weinberg N, Dor Y, Levenberg S (2012) Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS ONE 7(7):e40741
Song S, Roy S (2016) Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices. Biotechnol Bioeng 113:1381–1402
Silva PN, Green BJ, Altamentova SM, Rocheleau JV (2013) A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab Chip 13:4374–4384
Santos GJ, Oliveira CAM, Boschero AC, Rezende LF (2011) CNTF protects MIN6 cells against apoptosis induced by Alloxan and IL-1β through downregulation of the AMPK pathway. Cell Signal 23(10):1669–1676
Jorns A, Munday R, Tiedge M, Lenzen S (1997) Comparative toxicity of alloxan, N-alkylalloxans and ninhydrin to isolated pancreatic islets in vitro. J Endocrinol 155:283–293
Ighodaro OM, Adeosun AM, Akinloye OA (2017) Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 53(6):365–374
Gharami K, Das M, Das S (2015) Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int 89:51–62
Das M, Das S (2019) Docosahexaenoic acid (DHA) induced morphological differentiation of astrocytes is associated with transcriptional upregulation and endocytosis of β2-AR. Mol Neurobiol 56:2685–2702
Das M, Das S (2016) Identification of cytotoxic mediators and their putative role in the signaling pathways during docosahexaenoic acid (DHA)-induced apoptosis of cancer cells. Apoptosis 21(12):1408–1421
Pujari P, Roy R (2012) Dietary polyunsaturated fatty acids alleviate D-galcatosamine induced hepatitis by regulating cytokine production. Int J Integr Biol 13:24–29
Kamat SG, Roy R (2015) Evaluation of fish oils in amelioration of diabetes-induced tissue dam-ages in mice (Mus musculus). South Asian J Exp Biol 5(1):32–40
Kamat SG, Roy R (2016) Evaluation of the effect of n3 PUFA rich dietary fish oils on lipid profile and membrane fluidity in alloxan induced diabetic mice (Mus musculus). Mol Cell Biochem 416:117–129
Gao J, Xiao H, Li J, Guo X, Cai W, Li D (2019) N-3 polyunsaturated fatty acids decrease long-term diabetic risk of offspring of gestational diabetes rats by postponing shortening of hepatic telomeres and modulating liver metabolism. Nutrients 11(7):1699
Gao C, Liu Y, Gan Y, Bao W, Peng X, Xing Q, Gao H, Lai J, Liu L, Wang Z, Yang Y (2020) Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a Meta-analysis of randomized controlled trials. Lipids Health Dis 19(1):87
Gao H, Geng T, Huang T, Zhao Q (2017) Fish oil supplementation and insulin sensitivity: a systematic review and meta-analysis. Lipids Health Dis 16(1):131
Bi X, Li F, Liu S, Jin Y, Zhang X, Yang T, Dai Y, Li X, Zhao AZ (2017) ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Investig 127(5):1757–1771
Suresh Y, Das UN (2006) Differential effect of saturated, monounsaturated, and polyunsaturated fatty acids on alloxan-induced diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 74:199–213
Rebuffat SA, Sidot E, Guzman C, Azay-Milhau J, Jover B, Lajoix AD, Peraldi-Roux S (2018) Adipose tissue derived-factors impaired pancreatic β-cell function in diabetes. Biochim Biophys Acta Mol Basis Dis 1864(10):3378–3387
Barlow J, Solomon TPJ (2019) Conditioned media from contracting skeletal muscle potentiates insulin secretion and enhances mitochondrial energy metabolism of pancreatic beta-cells. Metabolism 91:1–9
Mizgier ML, Fernández-Verdejo R, Cherfan J, Pinget M, Bouzakri K, Galgani JE (2019) Insights on the role of putative muscle-derived factors on pancreatic beta cell function. Front Physiol 10:1024
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y (1993) Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36:1139–1145
Kameswararao B, Kesavulu MM, Apparao C (2003) Evaluation of antidiabetic effect of Momordica cymbalaria fruit in alloxan-diabetic rats. Fitoterapia 74(1–2):7–13
Pari L, Saravanan G (2002) Antidiabetic effect of Cogent db, a herbal drug in alloxan-induced diabetes mellitus. Comp Biochem Physiol C: Toxicol Pharmacol 131:19–25
Borg LA, Eide SJ, Andersson A, Hellerström C (1979) Effects in vitro of alloxan on the glucose metabolism of mouse pancreatic B-cells. Biochem J 182(3):797–802
Malaisse WJ, Malaisse-Lagae F, Sener A, Pipeleers DG (1982) Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci USA 79(3):927–930
Jeromson S, Gallagher IJ, Galloway SD, Hamilton DL (2015) Omega-3 fatty acids and skeletal muscle health. Mar Drugs 13(11):6977–7004
Todorčević M, Hodson L (2016) The effect of marine derived n-3 fatty acids on adipose tissue metabolism and function. J Clin Med 5(1):3
Verlengia R, Gorjão R, Kanunfre CC, Bordin S, Martins de Lima T, Martins EF, Newsholme P, Curi R (2004) Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids 39:857–864
Rho H, Lee J, Kim H, Park B, Park J (2000) Protective mechanism of glucose against alloxan-induced β-cell damage: pivotal role of ATP. Exp Mol Med 32:12–17
Baynes HW, Mideksa S, Ambachew S (2018) The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 7(2):81–87
Wang X, Chan CB (2015) n-3 polyunsaturated fatty acids and insulin secretion. J Endocrinol 224(3):R97-106
Wei D, Li J, Shen M, Jia W, Chen N, Chen T, Su D, Tian H, Zheng S, Dai Y (2010) Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic β-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 59:471–478
Meglasson MD, Matschinsky FM (1986) Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev 2:163–214
Newgard CB, McGarry JD (1995) Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 64:689–719
Fan YY, McMurray DN, Ly LH, Chapkin RS (2003) Dietary(n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutri 133:1913–1920
Garattini S (2007) Long-chain n-3 fatty acids in lipid rafts: implications for anti-inflammatory effects. J Cardiovasc Med 8(Suppl 1):S30–S33
Jump DB (2002) The biochemistry of n-3 polyunsaturated fatty acids. J Bio Chem 277:8755–8758
Anderson BM, Ma DW (2009) Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis 8:33
Ohtsubo K, Takamatsu S, Gao C, Korekane H, Kurosawa TM, Taniguchi N (2013) N-glycosylation modulates the membrane sub-domain distribution and activity of glucose transporter 2 in pancreatic β cells. Biochem Biophys Res 434:346–351
Ferreira MR, Chicco A, Lombardo YB (2013) Dietary fish oil normalized glucose-stimulated insulin secretion in isolated pancreatic islets of dyslipemic rats through mechanisms involving glucose phosphorylation, peroxisome proliferator-activated receptor γ and uncoupling protein 2. Prostaglandins Leukot Essent Fatty Acids 89(1):31–38
Roy S, Leidal AM, Ye J, Ronen SM, Debnath J (2017) Autophagy-dependent shuttling of TBC1D5 controls plasma membrane translocation of GLUT1 and glucose uptake. Mol Cell 67:84–95
Shinde SR, Maddika S (2017) PTEN regulates glucose transporter recycling by impairing SNX27 retromer assembly. Cell Rep 21:1655–1666
Kahn BB, Charron MJ, Lodish HF, Cushman SW, Flier JS (1989) Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Investig 84:404–411
Herman MA, Kahn BB (2006) Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Investig 116:1767–1775
Rose AJ, Richter EA (2005) Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology 20:260–270
Shepherd PR, Kahn BB (1999) Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med 341:248–257
Graham TE, Kahn BB (2007) Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm Metab Res 39:717–721
Mohammad S, Taha A, Bamezai RN, Baquer NZ (2006) Modulation of glucose transporter (GLUT4) by vanadate and Trigonella in alloxan-diabetic rats. Life Sci 78(8):820–824
Camps, M., Castelló, A., Muñoz, P., Monfar, M., Testar, X., Palacín, M., Zorzano , A. (1992) Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J 282 (3), 765–772.
Osawa H, Printz RL, Whitesell RR, Granner DK (1995) Regulation of hexokinase II gene transcription and glucose phosphorylation by catecholamines, cyclic AMP and insulin. Diabetes 44(12):1426–1432
Henningsen C, Zahner G, Thaiss F (2003) High glucose induces type 1 hexokinase gene expression in isolated glomeruli of diabetic rats and in mesangial cells. Nephron Physiol 93(3):67–75
Wilcox G (2005) Insulin and insulin resistance. Clin Biochem Rev 26(2):19–39
Kumashiro N, Beddow SA, Vatner DF, Majumdar SK, Cantley JL, Guebre-Egziabher F, Fat I, Guigni B, Jurczak MJ, Birkenfeld AL, Kahn M, Perler BK, Puchowicz MA, Manchem VP, Bhanot S, Still CD, Gerhard GS, Petersen KF, Cline GW, Shulman GI, Samuel VT (2013) Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62(7):2183–2194
Vestergaard H, Lund S, Larsen FS, Bjerrum OJ, Pedersen O (1993) Glycogen synthase and phosphofructokinase protein and mRNA levels in skeletal muscle from insulin-resistant patients with non-insulin-dependent diabetes mellitus. J Clin Investig 91(6):2342–2350
Löfman M, Yki-Järvinen H, Parkkonen M, Lindström J, Koranyi L, Schalin-Jäntti C, Groop L (1995) Increased concentrations of glycogen synthase protein in skeletal muscle of patients with NIDDM. Am J Physiol 269:E27-32
Huang X, Vaag A, Hansson M, Weng J, Laurila E, Groop L (2000) Impaired insulin-stimulated expression of the glycogen synthase gene in skeletal muscle of type 2 diabetic patients is acquired rather than inherited. J Clin Endocrinol Metab 85(4):1584–1590
Funding
The work was financially supported by Science and Engineering Research Board, New Delhi, India (SERB-NPDF scheme, File no. PDF/2017/000989 and EMR/2017/004290).
Author information
Authors and Affiliations
Contributions
The authors declare that this work was done by the authors named in this article. MD is a National postdoctoral fellow from the Science and Engineering Research Board, New Delhi, India (File no. PDF/2017/000989) was involved in study design, experimentation, data collection, writing the manuscript. Ramaballav Roy and Arnab Banerjee were involved in study design and reviewing the data and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This work does not include any human blood/tissue sample and/or animals.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11010_2022_4424_MOESM1_ESM.tif
Supplementary file1 (TIF 593 kb) Supplementary Figure 1 Analysis of differentiation of fat cells or muscle cells. C2C12 muscle cells were treated with 10% FBS and 2% horse serum for 5 days or 3T3 fat cells were treated with 500 µM IBMX, 0.25 µM dexamethasone, and 10 µg/ml insulin for 60 h followed by insulin treatment for 8 days for differentiation (Diff) of cells. Relative mRNA expression of (i) MyoG, (ii) MyH2 in (A) muscle cells and (i) Cebp-a, (ii) PPAR-g in (B) fat cells represented as histogram expressed as mean ± SEM of four per group, normalized with 18S rRNA. ɸ represents p<0.001 indicates statistical differences from undifferentiated cells (Undiff).
Rights and permissions
About this article
Cite this article
Das, M., Banerjee, A. & Roy, R. A novel in vitro approach to test the effectiveness of fish oil in ameliorating type 1 diabetes. Mol Cell Biochem 477, 2121–2132 (2022). https://doi.org/10.1007/s11010-022-04424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04424-1