Abstract
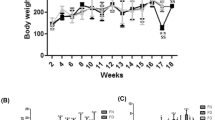
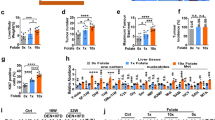
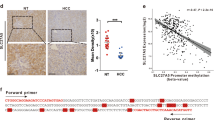
The current study evaluated the outcome of dietary folate modulations on the expression of tumor suppressor genes (TSGs) during developmental stages of hepatocellular carcinoma (HCC) in a Wistar rat model. In addition to dietary folate modulations, male rats were administered diethylnitrosamine (DEN) intraperitoneally once a week upto 18 weeks to induce HCC. Serum folate levels were found to be decreased and increased in folate deficiency (FD) and folate-oversupplemented (FO) groups respectively when compared to folate normal (FN) rats. Apoptosis was observed in FD in fibrosis and HCC stages. mRNA expression analysis by RT-PCR of TSGs (DPT, p16, RUNX3, RASSF1A and SOCS1) and protein expression by western blot (RASSF1A, RUNX3 and p16) depicted differential expression in FD and FO in various stages of HCC development. Bisulfite sequencing for p16 and RASSF1A promoter was performed. The promoter region of p16 gene was hypermethylated at 7th and that of RASSF1A was hypomethylated at 10th CpG in cirrhotic category in FD rats. Hyper and hypomethylation at 10th and 24th CpG respectively in RASSF1A promoter was observed in HCC category in both FD and FO groups. All TSGs showed differential expression at transcript and protein level. Increased expression of DPT, RASSF1A, SOCS1 and decreased expression of RUNX3 could be playing role in HCC development in FD rats. Reduced expression of RUNX3, RASSF1A and SOCS1 in HCC category was demonstrated in FO rats. Thus, the studied TSGs are differentially expressed with dietary folate modulations during the development of HCC in DEN-treated rat model and the promoter methylation might be a contributing mechanism under these conditions.









Similar content being viewed by others
Abbreviations
- FN:
-
Folate normal
- FD:
-
Folate deficiency
- FO:
-
Folate over-supplementation
- TSG:
-
Tumor suppressor genes
- DPT:
-
Dermatopontin
- RUNX3:
-
Runt-related transcription factor
- RASSF1A:
-
Ras association domain family member 1
- SOCS1:
-
Suppressor of cytokine signaling1
References
Correnti M et al (2018) Stemness features in liver cancer. Hepatoma Res 4:1–15
Cui L et al (2016) Plasma folate and vitamin B 12 levels in patients with hepatocellular carcinoma. Int J Mol Sci 17(7):1–10
Doll R (1983) Prospects for the prevention of cancer. Clin Radiol 34(6):609–623
Kinnaird A et al (2016) Metabolic control of epigenetics in cancer. Nat Rev Cancer 16(11):694–707
Kim YI (2006) Does a high folate intake increase the risk of breast cancer? Nutr Rev 6(10 Pt 1):468–475
Pieroth R (2018) Folate and its impact on cancer risk. Curr Nutr Rep 7(3):70–84
Su YH et al (2016) Folate deficient tumor microenvironment promotes epithelial-to-mesenchymal transition and cancer stem-like phenotypes. Oncotarget 7(22):33246–33256
Manshadi SD et al (2014) Folic acid supplementation promotes mammary tumor progression in a rat model. PLoS ONE 9(1):1–10
Song J et al (2000) Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res 60(12):5434–5440
Mason JB et al (2007) A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 16(7):1325–1329
Smith AD, Kim YI, Refsum H (2008) Is folic acid good for everyone? Am J Clin Nutr 87(3):517–533
Huang S, He X (2011) The role of microRNAs in liver cancer progression. Br J Cancer 104(2):235–240
Jin MJ et al (2009) Different histological types of non-small cell lung cancer have distinct folate and DNA methylation levels. Cancer Sci 100(12):2325–2330
Pogribny IP, James SJ (2002) De novo methylation of the p16INK4A gene in early preneoplastic liver and tumors induced by folate/methyl deficiency in rats. Cancer Lett 187(1–2):69–75
Kraunz KS et al (2006) Dietary folate is associated with p16 INK4A methylation in head and neck squamous cell carcinoma. Int J Cancer 119(7):1553–1557
Kk M et al (2007) Older age and dietary folate are determinants of genomic and p16-specific DNA methylation in mouse colon. J Nutr 137(7):1713–1717
Van Engeland M et al (2003) Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res 63(12):3133–3137
Bagnyukova TV et al (2008) Epigenetic downregulation of the suppressor of cytokine signaling 1 (Socs1) gene is associated with the STAT3 activation and development of hepatocellular carcinoma induced by methyl-deficiency in rats. Cell Cycle 7(20):3202–3210
Savini C et al (2019) Folate repletion after deficiency induces irreversible genomic and transcriptional changes in human papillomavirus type 16 (HPV16)-immortalzed human keratinocytes. Int J Mol Sci 20(5):1100
Busch EL et al (2018) Lifestyle factors, colorectal tumor methylation, and survival among African Americans and European Americans. Sci Rep 8(1):1–7
Bieri JG et al (1977) Report of the American institute of nutrition ad hoc committee on standards for nutritional studies. J Nutr 107(7):1340–1348
Bieri JG (1980) Second report of the ad hoc committee for nutritional studies on standards. J Nutr 110(8):1726
Lefkowitch JH (2007) Liver biopsy assessment in chronic hepatitis. Arch Med Res 38(6):634–643
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Pal A et al (2010) Aberrant methylation and associated transcriptional mobilization of Alu elements contributes to genomic instability in hypoxia. J Cell Mol Med 14(11):2646–2654
Fu Y, Chung F (2018) Oxidative stress and hepatocarcinogenesis. Hepatoma Res 4(39):1–8
Yu LX, Ling Y, Wang HY (2018) Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol 2(1):1–10
Tian Y et al (2013) Epigenetic regulation of hepatocellular carcinoma in non-alcoholic fatty liver disease. Semin Cancer Biol 23(6):471–482
Lind M, Johansson L (2016) High homocysteine and low folate plasma concentrations are associated with cardiovascular events but not bleeding during warfarin treatment. Clin Chem Lab Med 54(1):1981–1986
Achon M et al (2000) High-dose folic acid supplementation in rats: effects on gestation and the methionine cycle. Br J Nutr 83(2):177–183
Thakur S, Dev S (2014) Reduced expression of folate transporters in kidney of a rat model of folate oversupplementation. Genes Nutr 9(1):1–12
Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380:1450–1462
Seung Kew Y (2018) Molecular mechanism of hepatocellular carcinoma. Hepatoma Res 4:1–9
Mcmarhn KE (1984) Increased urinary folate excretion and decreased plasma folate levels in the rat after acute ethanol treatment. Alcohol Clin Exp Res 8(2):172–178
Novakovic P et al (2006) Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis 27(5):916–924
Huang RFS et al (1999) Biochemical and molecular roles of nutrients folate deficiency induces a cell cycle-specific apoptosis in HepG2 cells. J Nutr 129(1):25–31
Chern CL et al (2001) Folate deficiency-induced oxidative stress and apoptosis are mediated via homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-kappaB in human Hep G2 cells. Biomed Pharmacother 55(8):434–442
Marsillach J et al (2008) Moderately high folic acid supplementation exacerbates experimentally induced liver fibrosis in rats. Exp Biol Med (Maywood) 233(1):38–47
Fu Y et al (2014) DNA methylation-mediated silencing of matricellular protein dermatopontin promotes hepatocellular carcinoma metastasis by α3β1 integrin-Rho GTPase signaling. Oncotarget 5(16):6701–6715
Talaulikar VS (2011) Folic acid in obstetric practice : a review. Obstet Gynecol Surv 66(4):240–247
Ashkavand Z et al (2017) Metabolic reprogramming by folate restriction leads to a less aggressive cancer phenotype. Mol Cancer Res 15(2):189–200
Nishina SI et al (2011) Restored expression of the tumor suppressor gene RUNX3 reduces cancer stem cells in hepatocellular carcinoma by suppressing Jagged1-Notch signaling. Oncol Rep 26(3):523–531
Gou Y et al (2017) RUNX3 regulates hepatocellular carcinoma cell metastasis via targeting miR-186/E-cadherin/EMT pathway. Oncotarget 8(8):380–388
Wang S et al (2017) Aberrant methylation of RUNX3 is present in Aflatoxin B1-induced transformation of the L02R cell line. Toxicology 385:1–9
Yang B et al (2003) Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol 163(3):1101–1107
Kato A et al (2006) Aberrant DNA methylation of E-cadherin and p16 genes in rat lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine. Mol Carcinog 45(2):106–111
Esteller M et al (2002) Cancer epigenetics and methylation. Science 297(5588):1807–1808
Sanchez H et al (2017) High levels of circulating folate concentrations are associated with DNA methylation of tumor suppressor and repair genes p16, MLH1, and MGMT in elderly Chileans. Clin Epigenetics 9(74):1–11
Crott J et al (2008) Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. J Nutr Biochem 19(5):328–335
Shimizu K et al (2006) Aberrant methylation patterns of the Rassf1a gene in rat lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine. Mol Carcinog 45(2):112–117
Schagdarsurengin U et al (2003) Frequent epigenetic inactivation of the RASSF1A gene in hepatocellular carcinoma. Oncogene 22(12):1866–1871
Zhang YJ et al (2002) High frequency of promoter hypermethylation of RASSF1A and p16 and its relationship to aflatoxin B1-DNA adduct levels in human hepatocellular carcinoma. Mol Carcinog 35(2):85–92
Vineis P et al (2011) DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics 6(2):195–201
Pirouzpanah S et al (2015) Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB, BRCA1, and RASSF1A genes in breast cancer patients. J Mol Med 93(8):917–934
Aravalli RN, Steer CJ, Cressman ENK (2008) Molecular mechanisms of hepatocellular carcinoma. Hepatology 48(6):2047–2063
Yoshikawa H et al (2001) SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 28(1):29–35
Calvisi DF et al (2006) Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology 130(4):1117–1128
Okochi O et al (2003) Methylation-mediated silencing of SOCS-1 gene in hepatocellular carcinoma derived from cirrhosis. Clin Cancer Res 9(14):5295–5298
Author information
Authors and Affiliations
Contributions
JK and RS has designed the overall study. RS has conducted hands on experiments. TA has contributed in the acquisition, and analysis of data related to blotting experiments. Indian Council of Medical Research provided funding to the research scholar (RS).
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, R., Ali, T. & Kaur, J. Tumor suppressor genes are differentially regulated with dietary folate modulations in a rat model of hepatocellular carcinoma. Mol Cell Biochem 476, 385–399 (2021). https://doi.org/10.1007/s11010-020-03915-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03915-3