Abstract
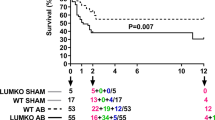
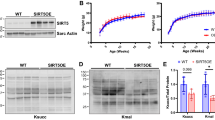
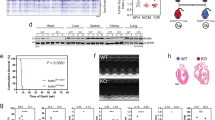
Heart failure (HF) is a functional lack of myocardial performance due to a loss of molecular control over increases in calcium and ROS, resulting in proteolytic degradative advances and cardiac remodeling. Mitochondria are the molecular powerhouse of cells, shifting the sphere of cardiomyocyte stability and performance. Functional mitochondria rely on the molecular abilities of safety factors such as TFAM to maintain physiological parameters. Mitochondrial transcription factor A (TFAM) creates a mitochondrial nucleoid structure around mtDNA, protecting it from mutation, inhibiting NFAT (ROS activator/hypertrophic stimulator), and transcriptionally activates Serca2a to decrease calcium mishandling. Calpain1 and MMP9 are proteolytic degratory factors that play a major role in cardiomyocyte decline in HF. Current literature depicts major decreases in TFAM as HF progresses. We aim to assess TFAM function against Calpain1 and MMP9 proteolytic activity and its role in cardiac remodeling. To this date, no publication has surfaced describing the effects of aortic banding (AB) as a surgical HF model in TFAM-TG mice. HF models were created via AB in TFAM transgenic (TFAM-TG) and C57BLJ-6 (WT) mice. Eight weeks post AB, functional analysis revealed a successful banding procedure, resulting in cardiac hypertrophy as observed via echocardiography. Pulse wave and color doppler show increased aortic flow rates as well as turbulent flow at the banding site. Preliminary results of cardiac tissue immuno-histochemistry of HF-control mice show decreased TFAM and compensatory increases in Serca2a fluorescent expression, along with increased Calpain1 and MMP9 expression. Protein, RNA, and IHC analysis will further assess TFAM-TG results post-banding. Echocardiography shows more cardiac stability and functionality in HF-induced TFAM-TG mice than the control counterpart. These findings complement our published in vitro results. Overall, this suggests that TFAM has molecular therapeutic potential to reduce protease expression.











Similar content being viewed by others
References
Mozaffarian D, Benjamin EJ, Go AS et al (2016) Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133:447–454
Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC (2014) Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Can J Physiol Pharmacol 92:583–591
Kunkel GH, Chaturvedi P, Tyagi SC (2015) Epigenetic revival of a dead cardiomyocyte through mitochondrial interventions. Biomol Concepts 2016 ‘Mitochondrial pathways to cardiac recovery: TFAM’. Heart Fail Rev 21:499–517
Lauritzen KH, Kleppa L, Aronsen JM et al (2015) Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol 309:H434–H449
Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236
Patterson C, Portbury A, Schisler JC, Willis MS (2011) Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ Res 109:453–462
Chen M, Won DJ, Krajewski S, Gottlieb RA (2002) Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277:29181–29186
Wanichawan P, Hafver TL, Hodne K, Aronsen JM, Ida et al (2014) Molecular basis of calpain cleavage and inactivation of the sodium-calcium exchanger 1 in heart failure. J Biol Chem 289:33984–33998
Yang DC, Ma ST, Tan Y et al (2010) Imbalance of matrix metalloproteinases/tissue inhibitor of metalloproteinase-1 and loss of fibronectin expression in patients with congestive heart failure. Cardiology 116:133–141
Williams CR, Gooch JL (2014) Calcineurin abeta regulates NADPH oxidase (Nox) expression and activity via nuclear factor of activated T cells (NFAT) in response to high glucose. J Biol Chem 289:4896–4905
Chang TH, Chen MC, Chang JP et al (2016) Exploring regulatory mechanisms of atrial myocyte hypertrophy of mitral regurgitation through gene expression profiling analysis: role of NFAT in cardiac hypertrophy. PLoS ONE 11:e0166791
Houser SR, Molkentin JD (2008) Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci Signal 1:pe31
Mujumdar VS, Smiley LM, Tyagi SC (2001) Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol 79:277–286
Prathipati P, Metreveli N, Nandi SS, Tyagi SC, Mishra PK (2016) Ablation of matrix metalloproteinase-9 prevents cardiomyocytes contractile dysfunction in diabetics. Front Physiol 7:93
Mishra PK, Givvimani S, Chavali V, Tyagi SC (2013) Cardiac matrix: a clue for future therapy. Biochim Biophys Acta 1832:2271–2276
Watanabe A, Arai M, Koitabashi N et al (2011) Mitochondrial transcription factors TFAM and TFB2M regulate Serca2 gene transcription. Cardiovasc Res 90:57–67
Fujino T, Ide T, Yoshida M et al (2012) Recombinant mitochondrial transcription factor A protein inhibits nuclear factor of activated T cells signaling and attenuates pathological hypertrophy of cardiac myocytes. Mitochondrion 12:449–458
Ikeuchi M, Matsusaka H, Kang D et al (2005) Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112:683–690
Kunkel GH, Chaturvedi P, Theilen N, Nair R, Tyagi SC (2017) Mechanism of TFAM mediated cardiomyocyte protection. Can J Physiol Pharmacol 96(2):173–181
Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chein KR (1991) Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA 88:8277–8281
Veeraveedu PT, Sanada S, Okuda K, Fu HY et al (2017) Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem Pharmacol 138:73–80
Ikeda M, Ide T, Fujino T et al (2015) Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS ONE 10:e0119687
Givvimani S, Tyagi N, Sen U et al (2010) MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch Physiol Biochem 116:63–72
Cox MJ, Sood HS, Hunt MJ, Chandler D, Henegar JR, Aru JM, Tyagi SC (2002) Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol 282:H1197–H1205
Lamparter S, Maisch B (2000) Significance of matrix metalloproteinases in cardiovascular diseases. Z Kardiol 89:949–957
Wang J, Wang Q, Watson LJ, Jones SP, Epstein PN (2011) Cardiac overexpression of 8-oxoguanine DNA glycosylase 1 protects mitochondrial DNA and reduces cardiac fibrosis following transaortic constriction. Am J Physiol Heart Circ Physiol 301:H2073–H2080
Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS (2009) Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol 2:686–694
Acknowledgements
This study was supported by the following grants from the US National Institute of Health; NIH F31 Grant 1F31HL132527-01 to GHK, and NIH grant HL 74185 to SCT. Thank you to Savanna C. Roberts of the University of Louisville English Department for editing this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim that there is no conflict of interest associated with this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2018_3459_MOESM1_ESM.pptx
This image exhibits the absorption of dihydroethidium by cardiac tissue. This stain is representative of superoxide stress and shows the positive conclusion of ROS accumulation in DNA with a bright nucleus. In the difference between the Sham WT and TFAM-TG models and the TAC or Aortic Banded animals we find a quantitative difference between these mice. Additionally, there is a significant difference in ROS concentration between the control and TFAM-TG banded mice. Supplementary material 1 (PPTX 998 KB)
Rights and permissions
About this article
Cite this article
Kunkel, G.H., Kunkel, C.J., Ozuna, H. et al. TFAM overexpression reduces pathological cardiac remodeling. Mol Cell Biochem 454, 139–152 (2019). https://doi.org/10.1007/s11010-018-3459-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3459-9