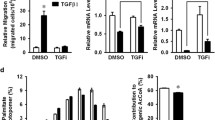
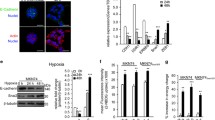
Abstract
The activation of phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) is critical for the induction of epithelial–mesenchymal transition (EMT) by growth factors, including insulin-like growth factor 1 (IGF-1). The activation of intracellular lipogenesis provides proliferative and survival signals for cancer cells. In this study, we investigated the connection between lipogenesis-related EMT processes and IGF-1-mediated PI3K p110 isoform activation in primary (SW480 cells) and metastatic (SW620) colon carcinoma cells. We also examined the underlying signaling pathway that promotes fatty acid synthesis in IGF-1-activated colon cancer cells. IGF-1 stimulation upregulated the expression of lipogenic enzymes as well as the activation of Nardilysin (N-arginine dibasic convertase, NRD1) and its downstream targets, a disintegrin and metalloproteases 10 (ADAM10) and ADAM17. The upregulation of the Lyn/Syk-mediated PI3K p110δ isoform in SW480 cells and the Lyn-dependent PI3K p110α isoform in SW620 cells triggered fatty acid production and cell motility in IGF-1-activated colon cancer cells. Pharmacological inhibition with A66 (PI3K p110α specific inhibitor) and CAL-101 (PI3K p110δ specific inhibitor) efficiently inhibited EMT in colon cancer cells by blocking the NRD1/ADAM family protein signaling pathway. Gene silencing of NRD1 and ADAM family proteins attenuated the generation of intracellular fatty acid and the migratory activity of colon cancer cells. Our results suggest that the different isoforms of the PI3K p110 subunit could be therapeutic targets for primary and metastatic colon cancer and that regulation of the NRD1/ADAM signaling pathway controls lipogenesis-mediated EMT in IGF-1-stimulated colon cancer cells.







Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AceCS1:
-
Acetyl-CoA synthetase 1
- ACLY:
-
ATP-citrate lyase
- ACSL1:
-
Mammalian long-chain acyl-CoA synthetase 1
- ADAM:
-
A disintegrin and metalloproteinase
- α-SMA:
-
Alpha(α)-smooth muscle actin
- CRC:
-
Colorectal cancer
- EMT:
-
Epithelial–mesenchymal transition
- ERK:
-
Extracellular signal-regulated kinase
- FASN:
-
Fatty acid synthase
- IGF-1:
-
Insulin-like growth factor-1
- MMP:
-
Matrix metalloproteinase
- NRD1:
-
N-arginine dibasic convertase
- PI3K:
-
Phosphoinositide 3-kinase
References
Fürstenberger G, Senn HJ (2002) Insulin-like growth factors and cancer. Lancet Oncol 3:298–302
Baserga R, Peruzzi F, Reiss K (2003) The IGF-1 receptor in cancer biology. Int J Cancer 107:873–877
Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM (2010) IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology 151:3752–3763
Liu P, Kong F, Wang J, Lu Q, Xu H, Qi T et al (2015) Involvement of IGF-1 and MEOX2 in PI3K/Akt1/2 and ERK1/2 pathways mediated proliferation and differentiation of perivascular adipocytes. Exp Cell Res 331:82–96
LeRoith D, Roberts CT Jr (2003) The insulin-like growth factor system and cancer. Cancer Lett 195:127–137
Yu H, Rohan T (2000) Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst 92:1472–1489
Walsh LA, Damjanovski S (2011) IGF-1 increases invasive potential of MCF 7 breast cancer cells and induces activation of latent TGF-β1 resulting in epithelial to mesenchymal transition. Cell Commun Signal 9:10
Bader AG, Kang S, Zhao L, Vogt PK (2005) Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer 5:921–929
Samuels Y, Ericson K (2006) Oncogenic PI3K and its role in cancer. Curr Opin Oncol 18:77–82
Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY (2012) PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol 18:3745–3751
Bénistant C, Chapuis H, Roche S (2000) A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene 19:5083–5090
Uddin S, Hussain AR, Ahmed M, Abubaker J, Al-Sanea N, Abduljabbar A et al (2009) High prevalence of fatty acid synthase expression in colorectal cancers in Middle Eastern patients and its potential role as a therapeutic target. Am J Gastroenterol 104:1790–1801
Long XH, Mao JH, Peng AF, Zhou Y, Huang SH, Liu ZL (2013) Tumor suppressive microRNA-424 inhibits osteosarcoma cell migration and invasion via targeting fatty acid synthase. Exp Ther Med 5:1048–1052
Uddin S, Siraj AK, Al-Rasheed M, Ahmed M, Bu R, Myers JN et al (2008) Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. J Clin Endocrinol Metab 93:4088–4097
Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M (2008) Fatty acid synthase over expression is an indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma. J Urol 180:1137–1140
Meena AS, Sharma A, Kumari R, Mohammad N, Singh SV, Bhat MK (2013) Inherent and acquired resistance to paclitaxel in hepatocellular carcinoma: molecular events involved. PLoS ONE 8:e61524
Jiang L, Wang H, Li J, Fang X, Pan H, Yuan X et al (2014) Up-regulated FASN expression promotes transcoelomic metastasis of ovarian cancer cell through epithelial-mesenchymal transition. Int J Mol Sci 15:11539–11554
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Luo DX, Peng XH, Xiong Y, Liao DF, Cao D, Li L (2011) Dual role of insulin-like growth factor-1 in acetyl-CoA carboxylase-alpha activity in human colon cancer cells HCT-8: downregulating its expression and phosphorylation. Mol Cell Biochem 357:255–262
Zhang D, Bar-Eli M, Meloche S, Brodt P (2004) Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem 279:19683–19690
Mira E, Mañes S, Lacalle RA, Márquez G, Martínez-A C (1999) Insulin-like growth factor I-triggered cell migration and invasion are mediated by matrix metalloproteinase-9. Endocrinology 140:1657–1664
Saftig P, Reiss K (2011) The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur J Cell Biol 90:527–535
McCulloch DR, Akl P, Samaratunga H, Herington AC, Odorico DM (2004) Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res 10(1 Pt 1):314–323
Guo J, He L, Yuan P, Wang P, Lu Y, Tong F et al (2012) ADAM10 overexpression in human non-small cell lung cancer correlates with cell migration and invasion through the activation of the Notch1 signaling pathway. Oncol Rep 28:1709–1718
Ni SS, Zhang J, Zhao WL, Dong XC, Wang JL (2013) ADAM17 is overexpressed in non-small cell lung cancer and its expression correlates with poor patient survival. Tumour Biol 34:1813–1818
Kanda K, Komekado H, Sawabu T, Ishizu S, Nakanishi Y, Nakatsuji M et al (2012) Nardilysin and ADAM proteases promote gastric cancer cell growth by activating intrinsic cytokine signaling via enhanced ectodomain shedding of TNF-α. EMBO Mol Med 4:396–411
Park GB, Kim D, Kim YS, Kim JW, Sun H, Roh KH et al (2015) Regulation of ADAM10 and ADAM17 by sorafenib inhibits epithelial-to-mesenchymal transition in epstein-barr virus-infected retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 56:5162–5173
Leibovitz A, Stinson JC, McCombs WB 3rd, McCoy CE, Mazur KC, Mabry ND (1976) Classification of human colorectal adenocarcinoma cell lines. Cancer Res 36:4562–4569
Sujobert P, Bardet V, Cornillet-Lefebvre P, Hayflick JS, Prie N, Verdier F et al (2005) Essential role for the p110δ isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood 106:1063–1066
Hickey FB, Cotter TG (2006) BCR-ABL regulates phosphatidylinositol 3-kinase-p110γ transcription and activation and is required for proliferation and drug resistance. J Biol Chem 281:2441–2450
Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T et al (1994) Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med 179:1725–1729
Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM (1999) SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem 274:32662–32666
Oshima T, Akaike M, Yoshihara K, Shiozawa M, Yamamoto N, Sato T et al (2008) Clinicopathological significance of the gene expression of matrix metalloproteinase-7, insulin-like growth factor-1, insulin-like growth factor-2 and insulin-like growth factor-1 receptor in patients with colorectal cancer: insulin-like growth factor-1 receptor gene expression is a useful predictor of liver metastasis from colorectal cancer. Oncol Rep 20:359–364
Li ZJ, Ying XJ, Chen HL, Ye PJ, Chen ZL, Li G et al (2013) Insulin-like growth factor-1 induces lymphangiogenesis and facilitates lymphatic metastasis in colorectal cancer. World J Gastroenterol 19:7788–7794
Cao Z, Liu LZ, Dixon DA, Zheng JZ, Chandran B, Jiang BH (2007) Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal 19:1542–1553
Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV et al (1999) Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol 30:1128–1133
Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M et al (2011) mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res 71:3246–3256
Samani AA, Yakar S, LeRoith D, Brodt P (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47
Klein S, Levitzki A (2009) Targeting the EGFR and the PKB pathway in cancer. Curr Opin Cell Biol 21:185–193
Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J et al (2005) PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24:6465–6481
Yoon A, Hurta RA (2001) Insulin like growth factor-1 selectively regulates the expression of matrix metalloproteinase-2 in malignant H-ras transformed cells. Mol Cell Biochem 223:1–6
Smith TM, Gilliland K, Clawson GA, Thiboutot D (2008) IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol 128:1286–1293
Park JB, Lee CS, Jang JH, Ghim J, Kim YJ, You S et al (2012) Phospholipase signalling networks in cancer. Nat Rev Cancer 12:782–792
Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y, Long XH et al (2013) Inhibition of fatty acid synthase suppresses osteosarcoma cell invasion and migration via downregulation of the PI3K/Akt signaling pathway in vitro. Mol Med Rep 7:608–612
Liu S, Zhang W, Liu K, Ji B, Wang G (2015) Silencing ADAM10 inhibits the in vitro and in vivo growth of hepatocellular carcinoma cancer cells. Mol Med Rep 11:597–602
You B, Shan Y, Shi S, Li X, You Y (2015) Effects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinoma. Cancer Sci 106:1506–1514
Lee SB, Schramme A, Doberstein K, Dummer R, Abdel-Bakky MS, Keller S et al (2010) ADAM10 is upregulated in melanoma metastasis compared with primary melanoma. J Invest Dermatol 130:763–773
Lin HM, Chatterjee A, Lin YH, Anjomshoaa A, Fukuzawa R, McCall JL et al (2007) Genome wide expression profiling identifies genes associated with colorectal liver metastasis. Oncol Rep 17:1541–1549
Gavert N, Sheffer M, Raveh S, Spaderna S, Shtutman M, Brabletz T et al (2007) Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res 67:7703–7712
Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou HF et al (2012) ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol 40:1714–1724
Acknowledgments
This study was supported by the Basic Science Research Program of Ministry of Education (NRF-2015R1D1A1A01056672) and the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A2A01053732) through the National Research Foundation (NRF) of Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2017_3148_MOESM1_ESM.tif
Supplemental Figure 1. IGF-1-mediated Syk kinase activation regulates colon cancer cell migration. (A) Cells (2 × 105/6-well) were treated with 100 pg/ml IGF-1 for 24 h. Total protein was subjected to Western blot analysis with the indicated antibodies. (B-D) To inhibit Syk phosphorylation, cells were pre-exposed to 200 nM Syk inhibitor Bay 61-3606 for 2 h and then treated with 100 pg/ml IGF-1 for 24 h. Total cell lysates were immunoblotted with antibodies against (B) p-Syk (Tyr323), p-Syk (Tyr525/526), Syk, or p110δ; (C) NRD1, ADAM10, ADAM17, E-cadherin, N-cadherin, Vimentin, ZO-1, or α-SMA. Data are representative of three independent experiments. β-actin served as an internal control. (D) The migratory activity and invasiveness of SW620 cells were detected by the tumor transendothelial migration assay kit and the BME cell invasion assay kit, respectively, as described in the Materials and Methods section. Each value is the mean ± standard deviation of 3 determinations. *, p<0.05. **, p<0.05. Data are representative of three independent experiments. Supplementary material 1 (TIFF 9202 kb)
11010_2017_3148_MOESM2_ESM.tif
Supplemental Figure 2. The expression of intracellular lipogenic enzymes in IGF-1-treated colon cancer cells at various conditions. Total protein was subjected to Western blot analysis with antibodies against AceCS1, p-ACLY, ACLY, ACSL1, p-ACC, ACC, or FASN protein were performed. β-actin served as an internal control. (A) Cells were transfected with Lyn-siRNA (200 nM) or control-siRNA for 36 h prior to experiments and then treated with 100 pg/ml IGF-1 for 24 h. (B) Cells were pre-exposed to 10 μM PI3K/Akt inhibitor LY294002 for 2 h and then treated with 100 pg/ml IGF-1 for 24 h. (C) Cells were transfected with NRD1-siRNA (200 nM) or control-siRNA for 36 h prior to experiments and then 100 pg/ml IGF-1 for 24 h. Supplementary material 2 (TIFF 8192 kb)
11010_2017_3148_MOESM3_ESM.tif
Supplemental Figure 3. NRD1 silencing prevents EMT processes in IGF-1-treated colon cancer cells. (A) Cell motility was increased by IGF-1 as measured by a wound healing assay. Cells were wounded (0 h) and maintained for 24 h in complete medium. Dotted lines indicate the edges of the wounds. Wound closure (measured after 24 h) was faster in cells treated with control-siRNA than in those treated with NRD1-siRNA. (B and C) The migratory activity and invasiveness of SW480 (B) or SW620 (C) cells were detected by the tumor transendothelial migration assay kit and the BME cell invasion assay kit, respectively, as described in the Materials and Methods section. Each value is the mean ± standard deviation of three determinations. *, p<0.05. **, p<0.05. Data are representative of three independent experiments. Supplementary material 3 (TIFF 9545 kb)
11010_2017_3148_MOESM4_ESM.tif
Supplemental Figure 4. Intracellular triglyceride production of IGF-1-activated colon cancer cells. (A) Cells (2 × 105/6-well) were treated with 100 pg/ml IGF-1 for 24 h. (B) Cells were pre-exposed to 10 μM PI3K/Akt inhibitor LY294002 for 2 h and then treated with 100 pg/ml IGF-1 for 24 h. (C) Cells were transfected with ADAM10-siRNA (200 nM), ADAM17-siRNA (200 nM), or control-siRNA for 36 h prior to experiments and then treated with 100 pg/ml IGF-1 for 24 h. Post treatment lipid was extracted and total triglyceride was estimated. Accumulation of cellular triglyceride was assayed using a colorimetric assay according to the manufacturer’s instruction. The amount of triglyceride present in the samples may be determined from the standard curve, which obtained from the appropriate triglyceride standards. Each value is the mean ± standard deviation of 3 determinations. Data are representative of three independent experiments. Supplementary material 4 (TIFF 9815 kb)
11010_2017_3148_MOESM5_ESM.tif
Supplemental Figure 5. Intracellular cholesterol production of IGF-1-activated colon cancer cells. (A) Cells (2 × 105/6-well) were treated with 100 pg/ml IGF-1 for 24 h. (B) Cells were pre-exposed to 10 μM PI3K/Akt inhibitor LY294002 for 2 h and then treated with 100 pg/ml IGF-1 for 24 h. (C) Cells were transfected with ADAM10-siRNA (200 nM), ADAM17-siRNA (200 nM), or control-siRNA for 36 h prior to experiments and then treated with 100 pg/ml IGF-1 for 24 h. After post-treatment with IGF-1, lipid was extracted and then total cholesterol was estimated. Accumulation of cellular cholesterol was assayed using a colorimetric assay according to the manufacturer’s instruction. The amount of cholesterol present in the samples may be determined from the standard curve, which obtained from the appropriate cholesterol standards (in Kit). Each value is the mean ± standard deviation of 3 determinations. Data are representative of three independent experiments. Supplementary material 5 (TIFF 9804 kb)
Rights and permissions
About this article
Cite this article
Park, G.B., Kim, D. Insulin-like growth factor-1 activates different catalytic subunits p110 of PI3K in a cell-type-dependent manner to induce lipogenesis-dependent epithelial–mesenchymal transition through the regulation of ADAM10 and ADAM17. Mol Cell Biochem 439, 199–211 (2018). https://doi.org/10.1007/s11010-017-3148-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3148-0