Abstract
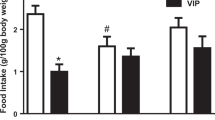
Vasoactive intestinal peptide (VIP) is a neurotransmitter with anorectic effect that acts in the hypothalamus to regulate food intake. Oxytocin is a neuropeptide produced in the hypothalamus that controls energy homeostasis and has an inhibitory role on food intake. Thus, the present study aims at verifying the role of oxytocin as a mediator of VIP on energy homeostasis. For this purpose, intracerebroventricular microinjection of oxytocin receptor antagonist (vasotocin, OVT) or vehicle (NaCl 0.9%) was carried out in male rats, and after 15 min, VIP or saline was microinjected. After 15 min of the second microinjection, food intake was evaluated or euthanasia was undertaken for blood collection. There was a reduction on food intake after VIP microinjection and the pretreatment with OVT partially reversed this effect. Hyperglycemia was observed after VIP microinjection, and pretreatment with OVT partially blocked this effect. Plasma corticosterone concentration was significantly increased after VIP or OVT. Plasma levels of free fatty acids were decreased by VIP, but not when VIP was microinjected after OVT. Thus, OVT partially reversed VIP-induced hypophagia and changes on plasma metabolic parameters, suggesting a role for oxytocin as a mediator of VIP effects on energy homeostasis.




Similar content being viewed by others
References
Said SI, Mutt V (1970) Polypeptide with broad biological activity: isolation from small intestine. Science 169:1217–1218
Taylor DP, Pert CB (1979) Vasoactive intestinal polypeptide: specific binding to rat brain membranes. Proc Natl Acad Sci USA 76:660–664
Alexander LD, Sander LD (1994) Vasoactive intestinal peptide stimulates ACTH and corticosterone release after injection into the PVN. Regul Pept 51:221–227
Ghourab S, Beale EK, Semjonous NM, Simpson KA, Martin NM, Ghatei MA, Bloom SR, Smith KL (2011) Intracerebroventricular administration of vasoactive intestinal peptide inhibits food intake. Regul Pept 172:8–15
Sims KB, Hoffman DL, Said SI, Zimmerman EA (1980) Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res 186:165–183
De Souza EB, Seifert H, Kuhar MJ (1985) Vasoactive intestinal peptide receptor localization in rat forebrain by autoradiography. Neurosci Lett 56:113–120
Mezey E, Kiss JZ (1985) Vasoactive intestinal peptide-containing neurons in the paraventricular nucleus may participate in regulating prolactin secretion. Proc Natl Acad Sci USA 82:245–247
Köves K, Arimura A, Görcs TG, Somogyvári-Vigh A (1991) Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology 54:159–169
Sheward WJ, Lutz EM, Harmar AJ (1995) The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience 67:409–418
Gerhold LM, Horvath TL, Freeman ME (2001) Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res 91:48–56
Falke N (1991) Modulation of oxytocin and vasopressin release from rat neurosecretosomes: the roles of VIP oxytocin and GABA. Neuropeptides 18:143–147
Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function and regulation. Physiol Rev 81:629–683
Olszewski PK, Klockars A, Schiöth HB, Levine AS (2010) Oxytocin as feeding inhibitor: maintaining homeostasis in consummatory behavior. Pharmacol Biochem Behav 97:47–54
Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T (2011) Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 3:1169–1177
Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE (2012) Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 302:E134–E144
Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schiöth HB, Levine AS (2010) Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology 151:4736–4744
Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG (1991) Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12:113–118
Arletti R, Benelli A, Bertolini A (1989) Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93
Arletti R, Benelli A, Bertolini A (1990) Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48:825–830
Uchôa ET, Silva LECM, Castro M, Antunes-Rodrigues J, Elias LL (2009) Hypothalamic oxytocin neurons modulate hypophagic effect induced by adrenalectomy. Horm Behav 56:532–538
Uchôa ET, Zahm DS, De Carvalho Borges B, Rorato R, Antunes-Rodrigues J, Elias LL (2013) Oxytocin projections to the nucleus of the solitary tract contribute to the increased meal-related satiety responses in primary adrenal insufficiency. Exp Physiol 98:1495–1504
Tachibana T, Saito S, Tomonaga S, Takagi T, Saito ES, Boswell T, Furuse M (2003) Intracerebroventricular injection of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibits feeding in chicks. Neurosci Lett 339:203–206
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordenates. Academic Press, San Diego
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24
Falholt K, Lund B, Falholt W (1973) An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin Chim Acta 46:105–111
Guillemin R, Clayton GW, Smith JD, Lipscomb HS (1958) Measurement of free corticosteroids in rat plasma: physiological validation of a method. Endocrinology 63:349–358
Nagai N, Kajikawa H, Sasaki T, Nagai K, Nakagawa H (1994) Hyperglycemic response to intracranial injection of vasoactive intestinal peptide. J Clin Biochem Nutr 17:29–34
Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E (2007) Glucose metabolism and catecholamines. Crit Care Med 35:S508–S518
Nunn N, Womack M, Dart C, Barrett-Jolley R (2011) Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr Neuropharmacol 9:262–277
Yee JR, Kenkel WM, Frijling JL, Dodhia S, Onishi KG, Tovar S, Saber MJ, Lewis GF, Liu W, Porges SW, Carter CS (2016) Oxytocin promotes functional coupling between paraventricular nucleus and both sympathetic and parasympathetic cardioregulatory nuclei. Horm Behav 80:82–91
Altszuler N, Hampshire J (1981) Oxytocin infusion increases plasma insulin and glucagon levels and glucose production and uptake in the normal dog. Diabetes 30:112–114
Vila G, Riedl M, Resl M, Van Der Lely AJ, Hofland LJ, Clodi M, Luger A (2009) Systemic administration of oxytocin reduces basal and lipopolysaccharide-induced ghrelin levels in healthy men. J Endocrinol 203:175–179
Camerino C (2009) Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 17:980–984
Richter WO, Robl H, Schwandt P (1989) Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10:333–335
van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, Sniderman A, Arner P (1999) Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem 274:18243–18251
Mirsky IA (1963) Effect of oxytocin, vasopressin, and related peptides on plasma free fatty acids. Am J Physiol 204:842–846
Itoh S, Hirota R, Katsuura G (1982) Effect of cholecystokinin octapeptide and vasoactive intestinal polypeptide on adrenocortical secretion in the rat. Jpn J Physiol 32:553–560
Windle RJ, Shanks N, Lightman SL, Ingram CD (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138:2829–2834
Olson BR, Drutarosky MD, Stricker EM, Verbalis JG (1991) Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol Regul Integr Comp Physiol 260:R448–R452
Olson BR, Drutarosky MD, Stricker EM, Verbalis JG (1991) Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129:785–791
Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG (2003) Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993:30–41
Blevins JE, Schwartz MW, Baskin DG (2004) Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96
Yosten GLC, Samson WK (2010) The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298:R1642–R1647
Lu XY, Barsh GS, Akil H, Watson SJ (2003) Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23:7863–7872
Acknowledgements
We would like to thank the Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico, Paraná, Brasil, for the Grant (Protocol Number 23088). ABM thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for Master fellowship. We also would like to thank Dr. Cláudia Bueno dos Reis Martinez (head of Laboratório de Ecofisiologia Animal, State University of Londrina) for the use of the Victor3™, PerkinElmer. Funding was provided by PRONEX-CNPq-Fundação Araucária-PR, for the Grant (Protocol Number 08586).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Martins, A.B., Garnica-Siqueira, M.C., Zaia, D.A.M. et al. Oxytocin participates on the effects of vasoactive intestinal peptide on food intake and plasma parameters. Mol Cell Biochem 437, 177–183 (2018). https://doi.org/10.1007/s11010-017-3106-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3106-x