Abstract
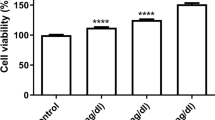
Myeloperoxidase (MPO) is able to promote several kinds of damage and is involved in mechanisms leading to various diseases such as atherosclerosis or cancers. An example of these damages is the chlorination of nucleic acids, which is considered as a specific marker of the MPO activity. Since 5-chlorocytidine has been recently shown in healthy donor plasmas, this study aimed at discovering if these circulating modified nucleosides could be incorporated into RNA and DNA and if their presence impacts the ability of enzymes involved in the incorporation, transcription, and translation processes. Experimentations, which were carried out in vitro with endothelial and prostatic cells, showed a large penetration of all chloronucleosides but an exclusive incorporation of 5-chlorocytidine into RNA. However, no incorporation into DNA was observed. This specific incorporation is accompanied by an important reduction of translation yield. Although, in vitro, DNA polymerase processed in the presence of chloronucleosides but more slowly than in control conditions, ribonucleotide reductase could not reduce chloronucleotides prior to the replication. This reduction seems to be a limiting step, protecting DNA from chloronucleoside incorporation. This study shows the capacity of transcription enzyme to specifically incorporate 5-chlorocytidine into RNA and the loss of capacity—complete or partial—of different enzymes, involved in replication, transcription or translation, in the presence of chloronucleosides. Questions remain about the long-term impact of such specific incorporation in the RNA and such decrease of protein production on the cell viability and function.







Similar content being viewed by others
References
Kundu JK, Surh YJ (2012) Emerging avenues linking inflammation and cancer. Free Radic Biol Med 52(9):2013–2037. doi:10.1016/j.freeradbiomed.2012.02.035
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77(5):598–625. doi:10.1189/jlb.1204697
Kundu JK, Surh YJ (2008) Inflammation: gearing the journey to cancer. Mutat Res 659(1–2):15–30. doi:10.1016/j.mrrev.2008.03.002
Freitas M, Baldeiras I, Proenca T, Alves V, Mota-Pinto A, Sarmento-Ribeiro A (2012) Oxidative stress adaptation in aggressive prostate cancer may be counteracted by the reduction of glutathione reductase. FEBS Open Bio 2:119–128. doi:10.1016/j.fob.2012.05.001
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49(11):1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
Klebanoff SJ (1999) Myeloperoxidase. Proc Assoc Am Physicians 111(5):383–389
Kettle AJ, Winterbourn CC (1997) Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep 3:3–15
Parker H, Albrett AM, Kettle AJ, Winterbourn CC (2012) Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol 91(3):369–376. doi:10.1189/jlb.0711387
Parker H, Winterbourn CC (2012) Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front Immunol 3:424. doi:10.3389/fimmu.2012.00424
Kato Y (2016) Neutrophil myeloperoxidase and its substrates: formation of specific markers and reactive compounds during inflammation. J Clin Biochem Nutr 58(2):99–104. doi:10.3164/jcbn.15-104
Ohshima H, Tazawa H, Sylla BS, Sawa T (2005) Prevention of human cancer by modulation of chronic inflammatory processes. Mutat Res 591(1–2):110–122. doi:10.1016/j.mrfmmm.2005.03.030
Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW (2001) Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci USA 98(21):11961–11966. doi:10.1073/pnas.211190298
Chapman AL, Senthilmohan R, Winterbourn CC, Kettle AJ (2000) Comparison of mono- and dichlorinated tyrosines with carbonyls for detection of hypochlorous acid modified proteins. Arch Biochem Biophys 377(1):95–100. doi:10.1006/abbi.2000.1744
Henderson JP, Byun J, Heinecke JW (1999) Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes produces 5-chlorocytosine in bacterial RNA. J Biol Chem 274(47):33440–33448
Stanley NR, Pattison DI, Hawkins CL (2010) Ability of hypochlorous acid and N-chloramines to chlorinate DNA and its constituents. Chem Res Toxicol 23(7):1293–1302. doi:10.1021/tx100188b
Masuda M, Suzuki T, Friesen MD, Ravanat JL, Cadet J, Pignatelli B, Nishino H, Ohshima H (2001) Chlorination of guanosine and other nucleosides by hypochlorous acid and myeloperoxidase of activated human neutrophils. Catalysis by nicotine and trimethylamine. J Biol Chem 276(44):40486–40496. doi:10.1074/jbc.M102700200
Gungor N, Godschalk RW, Pachen DM, Van Schooten FJ, Knaapen AM (2007) Activated neutrophils inhibit nucleotide excision repair in human pulmonary epithelial cells: role of myeloperoxidase. FASEB J 21(10):2359–2367. doi:10.1096/fj.07-8163com
Gungor N, Haegens A, Knaapen AM, Godschalk RW, Chiu RK, Wouters EF, van Schooten FJ (2010) Lung inflammation is associated with reduced pulmonary nucleotide excision repair in vivo. Mutagenesis 25(1):77–82. doi:10.1093/mutage/gep049
Castillo-Tong DC, Pils D, Heinze G, Braicu I, Sehouli J, Reinthaller A, Schuster E, Wolf A, Watrowski R, Maki RA, Zeillinger R, Reynolds WF (2014) Association of myeloperoxidase with ovarian cancer. Tumour Biol 35(1):141–148. doi:10.1007/s13277-013-1017-3
Roumeguere T, Delree P, Van Antwerpen P, Rorive S, Vanhamme L, de Ryhove Lde L, Serteyn D, Wespes E, Vanhaerverbeek M, Boudjeltia KZ (2012) Intriguing location of myeloperoxidase in the prostate: a preliminary immunohistochemical study. Prostate 72(5):507–513. doi:10.1002/pros.21452
Khalili M, Mutton LN, Gurel B, Hicks JL, De Marzo AM, Bieberich CJ (2010) Loss of Nkx3.1 expression in bacterial prostatitis: a potential link between inflammation and neoplasia. Am J Pathol 176(5):2259–2268. doi:10.2353/ajpath.2010.080747
Noyon C, Delporte C, Dufour D, Cortese M, Rousseau A, Poelvoorde P, Nève J, Vanhamme L, Zouaoui Boudjeltia K, Roumeguère T, Van Antwerpen P (2016) Validation of a sensitive LC/MSMS method for chloronucleoside analysis in biological matrixes and its applications. Talanta 154:322–328. doi:10.1016/j.talanta.2016.03.087
Moguilevsky N, Garcia-Quintana L, Jacquet A, Tournay C, Fabry L, Pierard L, Bollen A (1991) Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur J Biochem 197(3):605–614
Van Antwerpen P, Moreau P, Zouaoui Boudjeltia K, Babar S, Dufrasne F, Moguilevsky N, Vanhaeverbeek M, Ducobu J, Neve J (2008) Development and validation of a screening procedure for the assessment of inhibition using a recombinant enzyme. Talanta 75(2):503–510. doi:10.1016/j.talanta.2007.11.040
Ravanat JL, Douki T, Duez P, Gremaud E, Herbert K, Hofer T, Lasserre L, Saint-Pierre C, Favier A, Cadet J (2002) Cellular background level of 8-oxo-7,8-dihydro-2′-deoxyguanosine: an isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis 23(11):1911–1918
Cory JG, Mansell MM (1975) Comparison of the cytidine 5′-diphosphate and adenosine 5′-diphosphate reductase activities of mammalian ribonucleotide reductase. Cancer Res 35(9):2327–2331
Cory JG, Mansell MM (1975) Studies on Mammalian ribonucleotide reductase inhibition by pyridoxal phosphate and the dialdehyde derivatives of adenosine, adenosine 5′-monophosphate, and adenosine 5′-triphosphate. Cancer Res 35:390–396
Whiteman M, Spencer JP, Jenner A, Halliwell B (1999) Hypochlorous acid-induced DNA base modification: potentiation by nitrite: biomarkers of DNA damage by reactive oxygen species. Biochem Biophys Res Commun 257(2):572–576. doi:10.1006/bbrc.1999.0448
Whiteman M, Jenner A, Halliwell B (1997) Hypochlorous acid-induced base modifications in isolated calf thymus DNA. Chem Res Toxicol 10(11):1240–1246. doi:10.1021/tx970086i
Whiteman M, Jenner A, Halliwell B (1999) 8-Chloroadenine: a novel product formed from hypochlorous acid-induced damage to calf thymus DNA. Biomarkers 4(4):303–310. doi:10.1080/135475099230831
Badouard C, Masuda M, Nishino H, Cadet J, Favier A, Ravanat JL (2005) Detection of chlorinated DNA and RNA nucleosides by HPLC coupled to tandem mass spectrometry as potential biomarkers of inflammation. J Chromatogr B 827(1):26–31. doi:10.1016/j.jchromb.2005.03.025
Hawkins CL, Pattison DI, Davies MJ (2002) Reaction of protein chloramines with DNA and nucleosides: evidence for the formation of radicals, protein-DNA cross-links and DNA fragmentation. Biochem J 365(Pt 3):605–615. doi:10.1042/BJ20020363
Hawkins CL, Davies MJ (2002) Hypochlorite-induced damage to DNA, RNA, and polynucleotides: formation of chloramines and nitrogen-centered radicals. Chem Res Toxicol 15(1):83–92
Pattison DI, Davies MJ (2001) Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol 14(10):1453–1464
Nightingale ZD, Lancha AH Jr, Handelman SK, Dolnikowski GG, Busse SC, Dratz EA, Blumberg JB, Handelman GJ (2000) Relative reactivity of lysine and other peptide-bound amino acids to oxidation by hypochlorite. Free Radic Biol Med 29(5):425–433
Pattison DI, Davies MJ, Hawkins CL (2012) Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic Res 46(8):975–995. doi:10.3109/10715762.2012.667566
Takeshita J, Byun J, Nhan TQ, Pritchard DK, Pennathur S, Schwartz SM, Chait A, Heinecke JW (2006) Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages. J Biol Chem 281(6):3096–3104. doi:10.1074/jbc.M509236200
Henderson JP, Byun J, Takeshita J, Heinecke JW (2003) Phagocytes produce 5-chlorouracil and 5-bromouracil, two mutagenic products of myeloperoxidase, in human inflammatory tissue. J Biol Chem 278(26):23522–23528. doi:10.1074/jbc.M303928200
Asahi T, Kondo H, Masuda M, Nishino H, Aratani Y, Naito Y, Yoshikawa T, Hisaka S, Kato Y, Osawa T (2010) Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation. J Biol Chem 285(12):9282–9291. doi:10.1074/jbc.M109.054213
Valinluck V, Sowers LC (2007) Inflammation-mediated cytosine damage: a mechanistic link between inflammation and the epigenetic alterations in human cancers. Cancer Res 67(12):5583–5586. doi:10.1158/0008-5472.CAN-07-0846
Fox L, Dobersen MJ, Greer S (1983) Incorporation of 5-substituted analogs of deoxycytidine into DNA of herpes simplex virus-infected or -transformed cells without deamination to the thymidine analog. Antimicrob Agents Chemother 23(3):465–476
Vilpo JA, Vilpo LM (1991) Biochemical mechanisms by which reutilization of DNA 5-methylcytosine is prevented in human cells. Mutat Res 256(1):29–35
Fedeles BI, Freudenthal BD, Yau E, Singh V, Chang SC, Li D, Delaney JC, Wilson SH, Essigmann JM (2015) Intrinsic mutagenic properties of 5-chlorocytosine: a mechanistic connection between chronic inflammation and cancer. Proc Natl Acad Sci USA 112(33):E4571–E4580. doi:10.1073/pnas.1507709112
Sassa A, Kamoshita N, Matsuda T, Ishii Y, Kuraoka I, Nohmi T, Ohta T, Honma M, Yasui M (2013) Miscoding properties of 8-chloro-2′-deoxyguanosine, a hypochlorous acid-induced DNA adduct, catalysed by human DNA polymerases. Mutagenesis 28(1):81–88. doi:10.1093/mutage/ges056
Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, Nelson WG (1994) Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA 91(24):11733–11737
Valinluck V, Liu P, Kang JI Jr, Burdzy A, Sowers LC (2005) 5-halogenated pyrimidine lesions within a CpG sequence context mimic 5-methylcytosine by enhancing the binding of the methyl-CpG-binding domain of methyl-CpG-binding protein 2 (MeCP2). Nucleic Acids Res 33(9):3057–3064. doi:10.1093/nar/gki612
Kang JI Jr, Burdzy A, Liu P, Sowers LC (2004) Synthesis and characterization of oligonucleotides containing 5-chlorocytosine. Chem Res Toxicol 17(9):1236–1244. doi:10.1021/tx0498962
Acknowledgements
This study was supported by the grants from the Belgian Fund for Scientific Research (FRS-FNRS, Grant 34553.08 and 2.5018.12), the FER 2007 (Université Libre de Bruxelles), and David and Alice van Buuren Funds. C. Noyon and M. Cortese are research fellows of the FRS-FNRS, C. Delporte is a postdoctoral researcher funded by the FRS-FNRS, and L. Vanhamme is Research Director of the FRS-FNRS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Caroline Noyon and Thierry Roumeguère have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noyon, C., Roumeguère, T., Delporte, C. et al. The presence of modified nucleosides in extracellular fluids leads to the specific incorporation of 5-chlorocytidine into RNA and modulates the transcription and translation. Mol Cell Biochem 429, 59–71 (2017). https://doi.org/10.1007/s11010-016-2936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2936-2