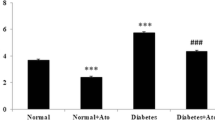
Abstract
Diabetes mellitus is a chronic condition that continues to increase in both incidence and prevalence. Renin–Angiotensin–Aldosterone System is one of the main modulators of chronic hyperglycaemia and, thus, its influence on tissues. Hyperglycaemia-induced oxidative stress is an important factor in diabetic cardiomyopathy. The present study was carried out on 24 adult male Wistar albino rats (8-week-old and with body masses of 190 ± 10 g). We evaluated the influence of acute administration of zofenopril on ex vivo myocardial function from rats with streptozotocin-induced diabetes mellitus, with a special emphasis on cardiodynamic and oxidative stress parameters in diabetic rat hearts. Rats were divided randomly into two groups (12 animals per group): control non-diabetic animals (C) were healthy rats perfused with 1.5 µM of zofenopril, and STZ-treated diabetic animals (DM) were diabetic animals perfused with 1.5 µM of zofenopril 4 weeks after the induction of diabetes. Our results demonstrated that diabetic rats are characterized by a depressed cardiac performance and that oxidative markers are related to alterations in cardiac function in rats with 4 weeks of STZ-induced diabetes. Additionally, the use of zofenopril as a monotherapy slightly diminished cardiac damage induced by chronic hyperglycaemia. However, long-term follow-up intervention trials are necessary to fully demonstrate the benefit of zofenopril in this context. A challenge for future investigations will be to identify the effects of chronic administration or combination therapy with angiotensin-converting enzyme inhibitors in various models of diabetes.




Similar content being viewed by others
Abbreviations
- LV:
-
Left ventricle
- RV:
-
Right ventricle
- HR:
-
Heart rate
- CPP:
-
Coronary perfusion pressure
- CF:
-
Coronary flow
- SEM:
-
Standard error mean
- SD:
-
Standard deviation
- DM:
-
Diabetes mellitus
- CAD:
-
Coronary artery disease
- HF:
-
Heart failure
- RAAS:
-
Renin–angiotensin system
- ATII:
-
Angiotensin II
- AT1:
-
Angiotensin II receptor type 1
- ACE:
-
Angiotensin-converting enzyme
- ACEI:
-
Angiotensin-converting enzymes inhibitor
- ARBs:
-
Angiotensin II receptor blockers
- ACE2:
-
Angiotensin-converting enzyme 2
- PLB:
-
Phospholamban
- SERCA:
-
Sarcoplasmic reticulum Ca2+ ATP-ase
- STZ:
-
Streptozotocin
- SH:
-
Sulfhydryl group
- dp/dt max:
-
Maximum rate of pressure development in LV
- dp/dt min:
-
Minimum rate of pressure development in LV
- SLVP:
-
Systolic left ventricle pressure
- DLVP:
-
Diastolic left ventricle pressure
- HR:
-
Heart rate
- CF:
-
Coronary flow
- ROS:
-
Reactive oxygen species
- NO2 − :
-
Nitrites
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthetase
- iNOS:
-
Inducible nitric oxide synthetase
- ·ONOO− :
-
Peroxynitrite
- O2−:
-
Superoxide anion radicals
- H2O2 :
-
Hydrogen peroxide
- TBARS:
-
Reactive thiobarbituric substances
- BP:
-
Blood pressure
References
Lim HS, MacFadyen RJ, Lip GY (2004) Diabetes mellitus, the renin–angiotensin–aldosterone system and the heart. Arch Intern Med 164(16):1737–1748
Singh VP, Le B, Khode R, Baker KM, Kumar R (2008) Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57(12):3297–3306
Kumar R, Yong QC, Thomas CM, Baker KM (2012) Intracardiac intracellular angiotensin system in diabetes. Am J Physiol Regul Integr Comp Physiol 302(5):R510–R517
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12(3):144–153
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C, ONTARGET Investigators (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358(15):1547–1559
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA, ALTITUDE Investigators (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367(23):2204–2213
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA, NEPHRON-D Investigators (2013) Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369(20):1892–1903
Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH (2013) Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomized trials. BMJ 28(346):f360
Barbato JC, Mulrow PJ, Shapiro JI, Franco-Saenz R (2002) Rapid effects of aldosterone and spironolactone in the isolated working rat heart. Hypertension 40(2):130–135
Lijnen PJ, van Pelt JF, Fagard RH (2012) Stimulation of reactive oxygen species and collagen synthesis by angiotensin II in cardiac fibroblasts. Cardiovasc Ther 30(1):e1–e8
Carnicelli V, Frascarelli S, Zucchi R (2011) Effect of acute and chronic zofenopril administration on cardiac gene expression. Mol Cell Biochem 352(1–2):301–307
Zhong Y, Ahmed S, Grupp IL, Matlib MA (2001) Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol 281(3):H1137–H1147
Vasanji Z, Dhalla NS, Netticadan T (2004) Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 261(1–2):245–249
Dhalla NS, Rangi S, Zieroth S, Xu YJ (2012) Alterations in sarcoplasmic reticulum and mitochondrial functions in diabetic cardiomyopathy. Exp Clin Cardiol 17(3):115–120
Subissi A, Evangelista S, Giachetti A (1999) Preclinical profile of zofenopril: an angiotensin converting enzyme inhibitor with peculiar cardioprotective properties. Cardiovasc Drug Rev 17(2):115–133
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115(25):3213–3223
Münzel T, Gori T, Keaney JF Jr, Maack C, Daiber A (2015) Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 36(38):2555–2564
DeForrest JM, Waldron TL, Krapcho J, Turk C, Rubin B, Powell JR, Cushman DW, Petrillo EW (1989) Preclinical pharmacology of zofenopril, an inhibitor of angiotensin I converting enzyme. J Cardiovasc Pharmacol 13(6):887–894
Tesch GH, Allen TJ (2007) Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 12:261–266
Auclair C, Voisin E (1985) Nitroblue tetrazolium reduction. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 123–132
Green LC, Wagnwr DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and (15 N) nitrate in biological fluids. Anal Biochem 126:131–138
Pick E, Keisari Y (1980) A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 38:161–170
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ambrosioni E (2007) Defining the role of zofenopril in the management of hypertension and ischemic heart disorders. Am J Cardiovasc Drugs 7(1):17–24
Working Party of the International Diabetes Federation (European Region) (2003) Hypertension in people with type 2 diabetes: knowledge-based diabetes-specific guidelines. Diabet Med 20:972–987
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS (1983) Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244(6):E528–E535
Zhao XY, Hu SJ, Li J, Mou Y, Chen BP, Xia Q (2006) Decreased cardiac sarcoplasmic reticulum Ca2+-ATPase activity contributes to cardiac dysfunction in streptozotocin-induced diabetic rats. J Physiol Biochem 62(1):1–8
Schaffer SW (1991) Cardiomyopathy associated with noninsulin-dependent diabetes. Mol Cell Biochem 107(1):1–20
Penpargkul S, Fein F, Sonnenblick EH, Scheuer J (1981) Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol 13(3):303–309
Howarth FC, Al-Sharhan R, Al-Hammadi A, Qureshi MA (2007) Effects of streptozotocin-induced diabetes on action potentials in the sinoatrial node compared with other regions of the rat heart. Mol Cell Biochem 300(1–2):39–46
Dhalla NS, Das PK, Sharma GP (1978) Subcellular basis of cardiaccontractile failure. J Mol Cell Cardiol 10:363–385
Nagareddy PR, Xia Z, McNeill JH, MacLeod KM (2005) Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol 289(5):H2144–H2152
Khanna S, Singh GB, Khullar M (2014) Nitric oxide synthases and diabetic cardiomyopathy. Nitric Oxide 1(43):29–34
West MB, Ramana KV, Kaiserova K, Srivastava SK, Bhatnagar A (2008) l-Arginine prevents metabolic effects of high glucose in diabetic mice. FEBS Lett 582(17):2609–2614
Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW, Khokha R, Penninger JM (2007) Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 75(1):29–39
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ristic, P., Srejovic, I., Nikolic, T. et al. The effects of zofenopril on cardiac function and pro-oxidative parameters in the streptozotocin-induced diabetic rat heart. Mol Cell Biochem 426, 183–193 (2017). https://doi.org/10.1007/s11010-016-2890-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2890-z