Abstract
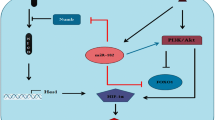
Given that HER2 serves as a putative target for therapy in HER2-positive breast cancer, intrinsic and/or acquired resistance to trastuzumab (T) has been proposed to be the major obstacle in treatments. In addition, chemoresistance is commonly attributed to increased antioxidant capacity. In that regard, we evaluated the effect of menadione (M) alone and/or its combination with trastuzumab on proliferation, intracellular GSH and ROS contents as well as HER2 and Notch1 signaling pathways in both trastuzumab-resistant (SKBR3R) and -sensitive SKBR3 (SKBR3S) cells. In spite of increased level of ROS and reduced level of GSH in M-treated SKBR3S cells, M-treated SKBR3R cells showed a decreased content of ROS and GSH compared to untreated cells. However, M/T co-treatment of SKBR3 cells indicated no effect on ROS content, while decreased the level of GSH compared to untreated control cells. Based on the extent of apoptosis, colony formation and wound healing assays, M alone, and/or in combination with T had a stronger inhibitory effect on proliferation of SKBR3R cells relative to SKBR3S cells. These effects might be due to the stronger effects of M and/or M/T on downregulation of p-Akt, Hes1, NICD, and upregulation of FOXO1 among SKBR3R cells relative to the sensitive SKBR3 cells. These findings would certainly shed light on some of the signaling factors involved in induction of trastuzumab resistance and would be of value in designing more efficient chemosensitization strategies.









Similar content being viewed by others
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- DCFH:
-
2′,7′-Dichlorofluorescein diacetate
- DTT:
-
Dithiothreitol
- AO/EtBr:
-
Acridine orange/Ethidium bromide
- GSH:
-
Reduced Glutathione
- MCF-7:
-
Human breast cancer cell line
- M:
-
Menadione
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAC:
-
N-Acetylcysteine
- NICD:
-
Notch1 intracellular domain
- PMS:
-
Phenazine methosulphate
- ROS:
-
Reactive oxygen species
- R or SKBR3R :
-
Trastuzumab-resistant SKBR3 cells
- S or SKBR3S :
-
Trastuzumab-sensitive SKBR3 cells
- T:
-
Trastuzumab
- T/M:
-
Trastuzumab/menadione combination
References
Sørlie T et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 98(19):10869–10874
Eroles P et al (2012) Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev 38(6):698–707
Nahta R, Esteva FJ (2006) Herceptin: mechanisms of action and resistance. Cancer Lett 232(2):123–138
Romond EH et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Buzdar AU et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Nahta R et al (2006) Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3(5):269–280
Osipo C et al (2008) ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a γ-secretase inhibitor. Oncogene 27(37):5019–5032
Krebs LT et al (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14(11):1343–1352
Wang J et al (2011) Notch1 is involved in migration and invasion of human breast cancer cells. Oncol Rep 26(5):1295
Simmons MJ et al (2012) NOTCH1 inhibition in vivo results in mammary tumor regression and reduced mammary tumorsphere-forming activity in vitro. Breast Cancer Res 14(5):R126
Kim TH et al (2014) Silibinin induces cell death through reactive oxygen species-dependent downregulation of Notch-1/ERK/Akt signaling in human breast cancer cells. J Pharmacol Exp Ther 349(2):268–278
Benhar M, Engelberg D, Levitzki A (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 3(5):420–425
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival*. J Cell Physiol 192(1):1–15
Poli G et al (2004) Oxidative stress and cell signalling. Curr Med Chem 11(9):1163–1182
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updates 7(2):97–110
Matsura T et al (1999) Hydrogen peroxide-induced apoptosis in HL-60 cells requires caspase-3 activation. Free Radical Res 30(1):73–83
Yamakawa H et al (2000) Activation of caspase-9 and-3 during H2O2-induced apoptosis of PC12 cells independent of ceramide formation. Neurol Res 22(6):556–564
Yokomizo A et al (1995) Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res 55(19):4293–4296
Tareen B et al (2008) A 12 week, open label, phase I/IIa study using Apatone® for the treatment of prostate cancer patients who have failed standard therapy. Int J Med Sci 5(2):62
Akiyoshi T et al (2009) The potential of vitamin K3 as an anticancer agent against breast cancer that acts via the mitochondria-related apoptotic pathway. Cancer Chemother Pharmacol 65(1):143–150
Liao W, Wu F, Wu C (2000) Binary/ternary combined effects of vitamin K3 with other antitumor agents in nasopharyngeal carcinoma CG1 cells. Int J Oncol 17(2):323–331
Margolin KA et al (1995) Phase I study of mitomycin C and menadione in advanced solid tumors. Cancer Chemother Pharmacol 36(4):293–298
Tetef M et al (1995) Mitomycin C and menadione for the treatment of lung cancer: a phase II trial. Invest New Drugs 13(2):157–162
Vistica DT et al (1991) Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res 51(10):2515–2520
Chou T-C, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2’, 7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Meshkini A, Yazdanparast R (2008) Involvement of ERK/MAPK pathway in megakaryocytic differentiation of K562 cells induced by 3-hydrogenkwadaphnin. Toxicol In Vitro 22(6):1503–1510
Liang K et al (2003) Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2(11):1113–1120
Lowry OH et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Kinnula VL, Crapo JD (2004) Superoxide dismutases in malignant cells and human tumors. Free Radic Biol Med 36(6):718–744
Estrela JM, Ortega A, Obrador E (2006) Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43(2):143–181
Fang J, Nakamura H, Iyer A (2007) Tumor-targeted induction of oxystress for cancer therapy. J Drug Target 15(7–8):475–486
Timolati F et al (2006) Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation–contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol 41(5):845–854
Gordon LI et al (2009) Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem 284(4):2080–2087
Dogan I et al (2011) Inhibition of ErbB2 by herceptin reduces viability and survival, induces apoptosis and oxidative stress in Calu-3 cell line. Mol Cell Biochem 347(1–2):41–51
Aird KM et al (2012) ErbB1/2 tyrosine kinase inhibitor mediates oxidative stress-induced apoptosis in inflammatory breast cancer cells. Breast Cancer Res Treat 132(1):109–119
Victorino VJ et al (2014) Overexpression of HER-2/neu protein attenuates the oxidative systemic profile in women diagnosed with breast cancer. Tumor Biol 35(4):3025–3034
Seidman AD et al (2001) Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 19(10):2587–2595
Raff JP et al (2004) Phase II study of weekly docetaxel alone or in combination with trastuzumab in patients with metastatic breast cancer. Clin Breast Cancer 4(6):420–427
Boonstra J, Post JA (2004) Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337:1–13
Kim S-J et al (2005) Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic β-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 280(23):22297–22307
Boreddy SR, Pramanik KC, Srivastava SK (2011) Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3 K/AKT/FOXO pathway. Clin Cancer Res 17(7):1784–1795
Chakrabarty A et al (2013) Trastuzumab-resistant cells rely on a HER2-PI3 K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res 73(3):1190–1200
Mittal S et al (2009) Cooperation of Notch and Ras/MAPK signaling pathways in human breast carcinogenesis. Mol Cancer 8(1):128
Efferson CL et al (2010) Downregulation of Notch pathway by a γ-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res 70(6):2476–2484
Meurette O et al (2009) Notch activation induces Akt signaling via an autocrine loop to prevent apoptosis in breast epithelial cells. Cancer Res 69(12):5015–5022
Acknowledgments
The authors appreciate the financial support of this investigation by the Research Council of University of Tehran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sajadimajd, S., Yazdanparast, R. Differential behaviors of trastuzumab-sensitive and -resistant SKBR3 cells treated with menadione reveal the involvement of Notch1/Akt/FOXO1 signaling elements. Mol Cell Biochem 408, 89–102 (2015). https://doi.org/10.1007/s11010-015-2485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2485-0