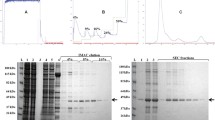
Abstract
Leishmania are protozoan pathogens of humans that exist as extracellular promastigotes in the gut of their sand fly vectors and as obligate intracellular amastigotes within phagolysosomes of infected macrophages. Between infectious blood meal feeds, sand flies take plant juice meals that contain sucrose and store these sugars in their crop. Such sugars are regurgitated into the sand fly anterior midgut where they impact the developing promastigote parasite population. In this report we showed that promastigotes of all Leishmania species secreted an invertase/sucrase enzyme during their growth in vitro. In contrast, neither L. donovani nor L. mexicana amastigotes possessed any detectable invertase activity. Importantly, no released/secreted invertase activity was detected in culture supernatants from either Trypanosoma brucei or Trypanosoma cruzi. Using HPLC, the L. donovani secretory invertase was isolated and subjected to amino acid sequencing. Subsequently, we used a molecular approach to identify the LdINV and LmexINV genes encoding the ~72 kDa invertases produced by these organisms. Interestingly, we identified high fidelity LdINV-like homologs in the genomes of all Leishmania sp. but none were present in either T. brucei or T. cruzi. Northern blot and RT-PCR analyses showed that these genes were developmentally/differentially expressed in promastigotes but not amastigotes of these parasites. Homologous transfection studies demonstrated that these genes in fact encoded the functional secretory invertases produced by these parasites. Cumulatively, our results suggest that these secretory enzymes play critical roles in the survival/growth/development and transmission of all Leishmania parasites within their sand fly vector hosts.








Similar content being viewed by others
References
UNICEF/UNDP/World Bank/World Health Organization (2010) Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. UNICEF/UNDP/World Bank/World Health Organization, Geneva
Handman E (2001) Leishmaniasis: current status of vaccine development. Clin Microbiol Rev 14:229–243
Sacks DL, Perkins PV (1984) Identification of an infective stage of Leishmania promastigotes. Science 223:1417–1419
Sacks D, Kamhawi S (2001) Molecular aspects of parasite–vector and vector–host interactions in leishmaniasis. Annu Rev Microbiol 55:453–483
Bates PA, Rogers ME (2004) New insights into the developmental biology and transmission mechanisms of Leishmania. Curr Mol Med 4:601–609
Kamhawi S (2006) Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol 22:439–445
Jacobson RL, Studentsky L, Schlein Y (2007) Glycolytic and chitinolytic activities of Phlebotomus papatasi (diptera: Psychodidae) from diverse ecological habitats. Folia Parasitol (Praha) 54:301–309
Blum JJ, Opperdoes FR (1994) Secretion of sucrase by Leishmania donovani. J Eukaryot Microbiol 41:228–231
Gontijo NF, Melo MN, Riani EB, Almeida-Silva S, Mares-Guia ML (1996) Glycosidases in Leishmania and their importance for Leishmania in phlebotomine sandflies with special reference to purification and characterization of a sucrase. Exp Parasitol 83:117–124
Stiles JK, Hicock PI, Shah PH, Meade JC (1999) Genomic organization, transcription, splicing and gene regulation in Leishmania. Ann Trop Med Parasitol 93:781–807
Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR et al (2014) Pfam: the protein families database. Nucleic Acids Res 42:D222–D230
Alberto F, Bignon C, Sulzenbacher G, Henrissat B, Czjzek M (2004) The three-dimensional structure of invertase (beta-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J Biol Chem 279:18903–18910
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Gerber LD, Kodukula K, Udenfriend S (1992) Phosphatidylinositol glycan (PI-G) anchored membrane proteins. Amino acid requirements adjacent to the site of cleavage and PI-G attachment in the COOH-terminal signal peptide. J Biol Chem 267:12168–12173
Teasdale RD, Jackson MR (1996) Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Annu Rev Cell Dev Biol 12:27–54
Borst P (1986) Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem 55:701–732
Grimaldi G Jr, Tesh RB (1993) Leishmaniases of the new world: current concepts and implications for future research. Clin Microbiol Rev 6:230–250
Zilberstein D, Dwyer DM (1984) Glucose transport in Leishmania donovani promastigotes. Mol Biochem Parasitol 12:327–336
Zilberstein D, Dwyer DM, Matthaei S, Horuk R (1986) Identification and biochemical characterization of the plasma membrane glucose transporter of Leishmania donovani. J Biol Chem 261:15053–15057
Barros VC, Oliveira JS, Melo MN, Gontijo NF (2006) Leishmania amazonensis: chemotaxic and osmotaxic responses in promastigotes and their probable role in development in the phlebotomine gut. Exp Parasitol 112:152–157
Burchmore RJ, Rodriguez-Contreras D, McBride K, Merkel P, Barrett MP, Modi G et al (2003) Genetic characterization of glucose transporter function in Leishmania mexicana. Proc Natl Acad Sci USA 100:3901–3906
Tran KD, Rodriguez-Contreras D, Shinde U, Landfear SM (2012) Both sequence and context are important for flagellar targeting of a glucose transporter. J Cell Sci 125:3293–3298
Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R (2007) Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol 65:1006–1017
Singh A, Mandal D (2011) A novel sucrose/H+ symport system and an intracellular sucrase in Leishmania donovani. Int J Parasitol 41:817–826
Bates PA, Hermes I, Dwyer DM (1990) Golgi-mediated post-translational processing of secretory acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol 39:247–255
Shakarian AM, Joshi MB, Ghedin E, Dwyer DM (2002) Molecular dissection of the functional domains of a unique, tartrate-resistant, surface membrane acid phosphatase in the primitive human pathogen Leishmania donovani. J Biol Chem 277:17994–18001
Silverman JM, Reiner NE (2011) Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol 13:1–9
Silverman JM, Reiner NE (2012) Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front Cell Infect Microbiol 1:26
Clayton C, Adams M, Almeida R, Baltz T, Barrett M, Bastien P et al (1998) Genetic nomenclature for Trypanosoma and Leishmania. Mol Biochem Parasitol 97:221–224
Debrabant A, Joshi MB, Pimenta PF, Dwyer DM (2004) Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int J Parasitol 34:205–217
McCarthy-Burke C, Bates PA, Dwyer DM (1991) Leishmania donovani: use of two different, commercially available, chemically defined media for the continuous in vitro cultivation of promastigotes. Exp Parasitol 73:385–387
Valenzuela JG, Pham VM, Garfield MK, Francischetti IM, Ribeiro JM (2002) Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol 32:1101–1122
Bates PA, Hermes I, Dwyer DM (1989) Leishmania donovani: immunochemical localization and secretory mechanism of soluble acid phosphatase. Exp Parasitol 68:335–346
Bates PA, Dwyer DM (1987) Biosynthesis and secretion of acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol 26:289–296
Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2014) Genbank. Nucleic Acids Res 42:D32–D37
Schneider A, McNally KP, Agabian N (1993) Splicing and 3′-processing of the tyrosine tRNA of Trypanosoma brucei. J Biol Chem 268:21868–21874
Fong D, Lee B (1988) Beta tubulin gene of the parasitic protozoan Leishmania mexicana. Mol Biochem Parasitol 31:97–106
Joshi MB, Dwyer DM (2007) Molecular and functional analyses of a novel class I secretory nuclease from the human pathogen, Leishmania donovani. J Biol Chem 282:10079–10095
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 7.43–7.45
Joshi MB, Hernandez Y, Owings JP, Dwyer DM (2012) Diverse viscerotropic isolates of Leishmania all express a highly conserved secretory nuclease during human infections. Mol Cell Biochem 361:169–179
Charest H, Zhang WW, Matlashewski G (1996) The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements located in the 3′-untranslated region. J Biol Chem 271:17081–17090
Ghedin E, Charest H, Zhang WW, Debrabant A, Dwyer D, Matlashewski G (1998) Inducible expression of suicide genes in Leishmania donovani amastigotes. J Biol Chem 273:22997–23003
Debrabant A, Ghedin E, Dwyer DM (2000) Dissection of the functional domains of the Leishmania surface membrane 3′-nucleotidase/nuclease, a unique member of the class I nuclease family. J Biol Chem 275:16366–16372
Zhang WW, Mendez S, Ghosh A, Myler P, Ivens A, Clos J et al (2003) Comparison of the A2 gene locus in Leishmania donovani and Leishmania major and its control over cutaneous infection. J Biol Chem 278:35508–35515
Agabian N (1990) Trans splicing of nuclear pre-mRNAs. Cell 61:1157–1160
Fernandes O, Murthy VK, Kurath U, Degrave WM, Campbell DA (1994) Mini-exon gene variation in human pathogenic Leishmania species. Mol Biochem Parasitol 66:261–271
Acknowledgments
This research was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). Dr. Todd Lyda was supported by an Intramural Research Training Award Post-doctoral Fellowship from the NIAID, NIH. Dr. Manju Joshi was supported by an appointment through the Oak Ridge Institute for Science and Education (ORISE), Research Specialist Program at NIH. The program is administered by ORISE through an inter-agency agreement between the US Department of Energy and the NIH. Mr. Andrew Y. Kelada and Mr. Joshua P. Owings were supported by Postbaccalaureate Intramural Research Training Awards from the NIAID, NIH. We thank Dr. Greg Matlashewski, Department of Microbiology and Immunology McGill University, Montreal, CA for providing the pKSNEO leishmanial expression vector; Dr. Buddy Ullman, Department of Biochemistry and Molecular Biology, Oregon Health Sciences University, Portland, OR for providing the L. donovani cosmid library used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Todd A. Lyda and Manju B. Joshi have contributed equally to the research reported in this study.
The nucleotide sequences reported in this paper have been submitted to the GENEBANK™/EBI Databank with accession number (KJ866149 and KJ866150).
Rights and permissions
About this article
Cite this article
Lyda, T.A., Joshi, M.B., Andersen, J.F. et al. A unique, highly conserved secretory invertase is differentially expressed by promastigote developmental forms of all species of the human pathogen, Leishmania . Mol Cell Biochem 404, 53–77 (2015). https://doi.org/10.1007/s11010-015-2366-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2366-6