Abstract
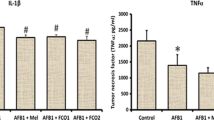
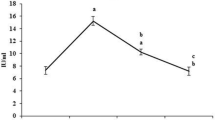
Aflatoxin-B1 (AFB1) intoxication is known to develop hepatocellular carcinoma (HCC). However, pathogenesis and diagnosis of AFB1-induced HCC remain undefined. This article describes histopathological progression versus kinetics of the placental glutathione S-transferase (GST-pi) expression and profiles of the antioxidant enzymes, pro-inflammatory cytokines, and proliferative cell nuclear antigen (PCNA) in the liver from the rats administered with two doses of 1 mg AFB1/kg b.w. Histopathologically, hepatocytes necrosis could be observed after 4 weeks of the AFB1 treatment, which subsequently developed into the well-defined foci of altered hepatocytes (FAH) at 10 weeks post-treatment stage. This was consistent with an increasing trend of GST-pi immunostaining especially in the liver foci as a function of FAH progression and thus, suggesting that GST-pi over expression may serve as a marker for AFB1-induced hepatocarcinogenesis. The liver from AFB1-treated rats showed significantly enhanced level of reactive oxygen species coinciding with the declined immunostaining for superoxide dismutase-1, a committed enzyme of the antioxidant pathway, in the FAH regions and also with declined activity of the other antioxidant enzymes. Concordantly, the liver from the AFB1-treated rats showed over expression of pro-inflammatory cytokines; TNF-α & IL-1α and a cell proliferative marker PCNA. These findings present histological characterization of AFB1-induced HCC development and provide evidence for activation of oxidative stress–pro-inflammatory pathway during hepatocarcinogenesis induced by AFB1 toxicity.





Similar content being viewed by others
References
Fink-Gremmels J (1999) Mycotoxins: their implications for human and animal health. Vet Q 21:115–120
Wang JS, Huang T, Su J, Liang F, Wei Z, Liang Y, Luo H, Kuang SY, Qian GS, Sun G, He X, Kensler TW, Groopman JD (2001) Hepatocellular carcinoma and aflatoxin exposure in Zhuqing Village, Fusui County, People’s Republic of China. Cancer Epidemiol Biomarkers Prev 10:143–146
Sherman M (2010) Epidemiology of hepatocellular carcinoma. Oncology 78(Suppl 1):7–10
Smela ME, Currier SS, Bailey EA, Essigmann JM (2001) The chemistry and biology of aflatoxin B (1): from mutational spectrometry to carcinogenesis. Carcinogenesis 22:535–545
Fujimoto Y, Hampton LL, Luo LD, Wirth PJ, Thorgeirsson SS (1992) Low frequency of p53 gene mutation in tumors induced by aflatoxin B1 in nonhuman primates. Cancer Res 52:1044–1046
Stanley LA, Mandel HG, Riley J, Sinha S, Higginson FM, Judah DJ, Neal GE (1999) Mutations associated with in vivo aflatoxin B1-induced carcinogenesis need not be present in the in vitro transformations by this toxin. Cancer Lett 137:173–181
Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M (2008) Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 14:156–165
Behne T, Copur MS (2012) Biomarkers for hepatocellular carcinoma. Int J Hepatol. doi:10.1155/2012/859076
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W (2000) Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407:390–395
Conklin KA (2004) Cancer chemotherapy and antioxidants. J Nutr 134:3201S–3204S
Hasegawa Y, Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, Amino N (2002) Decreased expression of glutathione peroxidase mRNA in thyroid anaplastic carcinoma. Cancer Lett 182:69–74
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Aquilano K, Vigilanza P, Rotilio G, Ciriolo MR (2006) Mitochondrial damage due to SOD1 deficiency in SH-SY5Y neuroblastoma cells: a rationale for the redundancy of SOD1. FASEB J 20:1683–1685
Ding WQ, Vaught JL, Yamauchi H, Lind SE (2004) Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: the potential importance of down-regulation of superoxide dismutase 1 expression. Mol Cancer Ther 3:1109–1117
Ha HL, Shin HJ, Feitelson MA, Yu DY (2010) Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol 16:6035–6043
Naiki-Ito A, Asamoto M, Hokaiwado N, Takahashi S, Yamashita H, Tsuda H, Ogawa K, Shirai T (2007) Gpx2 is an overexpressed gene in rat breast cancers induced by three different chemical carcinogens. Cancer Res 67:11353–11358
Brigelius-Flohé R, Kipp A (2009) Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 1790:1555–1568
Singh KB, Trigun SK (2013) Apoptosis of Dalton’s lymphoma due to in vivo treatment with emodin is associated with modulations of hydrogen peroxide metabolizing antioxidant enzymes. Cell Biochem Biophys 67:439–449
Dias MC, Rodrigues MAM, Reimberg MCH, Barbisan LF (2008) Protective effects of Ginkgo biloba against rat liver carcinogenesis. Chem Biol Interact 173:32–42
Ravinayagam V, Jaganathan R, Panchanadham S, Palanivelu S (2012) Potential antioxidant role of tridham in managing oxidative stress against aflatoxin-B(1)-induced experimental hepatocellular carcinoma. Int J Hepatol. doi:10.1155/2012/428373
Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F (1999) Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med 5:828–831
Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9:361–371
Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, Mantovani A, Dejana E (1990) Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res 50:4771–4775
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Qin Y, Ekmekcioglu S, Liu P, Duncan LM, Lizée G, Poindexter N, Grimm EA (2011) Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res 9:1537–1550
Afonso V, Santos G, Collin P, Khatib AM, Mitrovic DR, Lomri N, Leitman DC, Lomri A (2006) Tumor necrosis factor-alpha down-regulates human Cu/Zn superoxide dismutase 1 promoter via JNK/AP-1 signaling pathway. Free Radic Biol Med 41:709–721
Soffritti M, McConnell EE (1988) Liver foci formation during aflatoxin B1 carcinogenesis in the rat. Ann NY Acad Sci 534:531–540
Tsuda H, Fukushima S, Wanibuchi H, Morimura K, Nakae D, Imaida K, Tatematsu M, Hirose M, Wakabayashi K, Moore MA (2003) Value of GST-P positive preneoplastic hepatic foci in dose-response studies of hepatocarcinogenesis: evidence for practical thresholds with both genotoxic and nongenotoxic carcinogens. A review of recent work. Toxicol Pathol 31:80–86
Angsubhakorn S, Get-Ngern P, Miyamoto M, Bhamarapravati N (1990) A single dose-response effect of aflatoxin B1 on rapid liver cancer induction in two strains of rats. Int J Cancer 46:664–668
Singh S, Trigun SK (2010) Activation of neuronal nitric oxide synthase in cerebellum of chronic hepatic encephalopathy rats is associated with up-regulation of NADPH-producing pathway. Cerebellum 9:384–397
Abe R, Okano JI, Imamoto R, Fujise Y, Murawaki Y (2012) Sequential analysis of diethylnitrosamine-induced hepatocarcinogenesis in rats. Exp Ther Med 3:371–378
Mookerjee A, Basu JM, Majumder S, Chatterjee S, Panda GS, Dutta P, Pal S, Mukherjee P, Efferth T, Roy S, Choudhuri SK (2006) A novel copper complex induces ROS generation in doxorubicin resistant Ehrlich ascitis carcinoma cells and increases activity of antioxidant enzymes in vital organs in vivo. BMC Cancer 6:267
Singh S, Koiri RK, Trigun SK (2008) Acute and chronic hyperammonemia modulate antioxidant enzymes differently in cerebral cortex and cerebellum. Neurochem Res 33:103–113
Mahrotra A, Trigun SK (2012) Moderate grade hyperamonia induced concordant activation of antoxidant enzyme is associated with prevention of oxidative stress in brain slices. Neurochem Res 37:171–181
Koiri RK, Trigun SK (2011) Dimethyl sulfoxide activates tumor necrosis factor α-p53 mediated apoptosis and down regulates d-fructose-6-phosphate-2-kinase and lactate dehydrogenase-5 in Dalton’s lymphoma in vivo. Leuk Res 35:950–956
Pirisi M, Leutner M, Pinato DJ, Avellini C, Carsana L, Toniutto P, Fabris C, Boldorini R (2010) Reliability and reproducibility of the edmondson grading of hepatocellular carcinoma using paired core biopsy and surgical resection specimens. Arch Pathol Lab Med 134:1818–1822
Goodman ZD (2007) Neoplasma of liver. Mod Pathol 20:S49–S60
Essigmann JM, Croy RG, Bennett RA, Wogan GN (1982) Metabolic activation of aflatoxin B1: patterns of DNA adduct formation, removal, and excretion in relation to carcinogenesis. Drug Metab Rev 13:581–602
Bannasch P, Khoshkhou NI, Hacker HJ, Radaeva S, Mrozek M, Zillmann U, Kopp-Schneider A, Haberkorn U, Elgas M, Tolle T, Roggendorf M, Toskov I (1995) Synergistic hepatocarcinogenic effect of hepadnaviral infection and dietary aflatoxin B1 in woodchucks. Cancer Res 55:3318–3330
Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10:175–186
Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 711:193–201
Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38:96–109
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Tanaka M, Kogawa K, Nishihori Y, Kuribayashi K, Nakamura K, Muramatsu H, Koike K, Sakamaki S, Niitsu Y (1997) Suppression of intracellular Cu-Zn SOD results in enhanced motility and metastasis of Meth A sarcoma cells. Int J Cancer 73:187–192
Li N, Oberley TD, Oberley LW, Zhong W (1998) Overexpression of manganese superoxide dismutase in DU145 human prostate carcinoma cells has multiple effects on cell phenotype. Prostate 35:221–233
Ito N, Tamano S, Shirai T (2003) A medium-term rat liver bioassay for rapid in vivo detection of carcinogenic potential of chemicals. Cancer Sci 94:3–8
Yan W, Chen X (2006) GPX2, a direct target of p53, inhibits oxidative stress-induced apoptosis in a p53-dependent manner. J Biol Chem 281:7856–7862
Banning A, Kipp A, Schmitmeier S, Löwinger M, Florian S, Krehl S, Thalmann S, Thierbach R, Steinberg P, Brigelius-Flohé R (2008) Glutathione peroxidase 2 inhibits cyclooxygenase-2-mediated migration and invasion of HT-29 adenocarcinoma cells but supports their growth as tumors in nude mice. Cancer Res 68:9746–9753
Coussens LM, Werb Z (2009) Inflammation and cancer. Nature 420:860–867
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081
Johnson DG, Walker CL (2009) Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol 39:295–312
Stoimenov I, Helleday T (2009) PCNA on the crossroad of cancer. Biochem Soc Trans 37:605–613
Acknowledgments
The financial assistance in the form of a CSIR Project (P-25/321) to SKT is gratefully acknowledged. The award of CSIR JRF & SRF to BKM & the UGC JRF & SRF to KBS and the facilities of DST-FIST and UGC-CAS programs to the Department of Zoology are also acknowledged.
Conflict of interest
The authors declare no conflict of interest and equal contribution for this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, K.B., Maurya, B.K. & Trigun, S.K. Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin-B1 induced hepatocarcinogenesis in rat. Mol Cell Biochem 401, 185–196 (2015). https://doi.org/10.1007/s11010-014-2306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2306-x