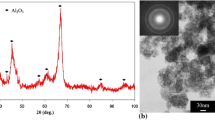
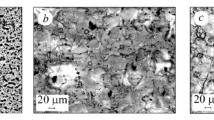
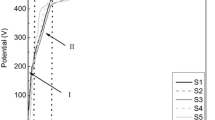
We study the influence of electrolyte composition and the parameters of the plasma-electrolytic oxidation of D16Т alloy on the corrosion resistance of synthesized coatings in a 3% NaCl aqueous solution. It was discovered that oxide-ceramic coatings decrease the density of corrosion currents by an order of magnitude as compared with the initial alloy. The lowest corrosion currents were recorded in coatings synthesized for the ratios of the cathodic and anodic components of current jc / ja equal to 15/10 and 10/10. It is shown that corrosion currents become stronger as the content of hydrogen peroxide (H2O2) in the electrolyte increase. This is explained by the increase in the sizes of through pores in the synthesized coatings. Through these pores corrosive media penetrate to the base material and, as a result, the rate of aluminum dissolution becomes higher.




Similar content being viewed by others
References
I. V. Suminov, P. М. Belkin, А. V. Épel’fel’d, V. B. Lyudin, B. L. Krit, and А. М. Borisov, Plasma-Electrolytic Modification of the Surfaces of Metals and Alloys [in Russian], Vol. 2, Tekhnosfera, Moscow (2011).
М. М. Student, V. М. Posuvailo, H. H. Veselivska, Ya. Ya. Sirak, and R. А. Yatsyuk, “Corrosion resistance of plasma-electrolytic layers on alloys and coatings of the Al–Cu–Mg system for various modes of heat treatment,” Fiz.-Khim. Mekh. Mater., 53, No 6, 42–47 (2017); English translation: Mater. Sci., 53, No. 6, 789–795 (2018).
T. Lampke, D. Meyer, G. Alisch, B. Wielage, H. Pokhmurska, M. Klapkiv, and M. Student, “Corrosion and wear behavior of alumina coatings obtained by various methods,” Fiz.-Khim. Mekh. Mater., 46, No. 5, 23–29 (2010); English translation: Mater. Sci., 46, No. 5, 591–598 (2011).
М. М. Student, V. V. Shmyrko, М. D. Klapkiv, І. M. Lyasota, and L. N. Dobrovol’ska, “Evaluation of the mechanical properties of combined metal-oxide-ceramic layers on aluminum alloys,” Fiz.-Khim. Mekh. Mater., 50, No. 2, 116–121 (2014); English translation: Mater. Sci., 50, No. 2, 290–295 (2014).
Y. Ma, X. Nie, D. O. Northwood, and H. Hu, “Systematic study of the electrolytic plasma oxidation process on a Mg alloy for corrosion protection,” Thin Solid Films, 49, 296–301 (2006).
R. F. Zhang, “Film formation in the second step of micro-arc oxidation on magnesium alloys,” Corr. Sci., 52, 1285–1290 (2010).
L. O. Snizhko, A. L. Yerohin, A. Pilkington, N. L. Gurevina, D. O. Misnyankina, A. Leyland, and A. Matthews, “Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions,” Electrochim. Acta, 49, 2085–2095 (2004).
M. Sieber, F. Simchen, R. Morgenstern, I. Scharf, and Th. Lampke, “Plasma electrolytic oxidation of high-strength aluminium alloys,” Substrate Effect on Wear and Corrosion Performance Metals, 8, No. 5, 1– 356 (2018); https://doi.org/10.3390/met8050356.
O. V. Bohoyavlenska, Improvement of the Technology of Microarc Oxidation of Aluminum Alloys in Alkaline Media [in Ukrainian], Author’s Abstr. of the Candidate-Degree Thesis (Engineering), Kharkiv (2010.
G.-H. Lv, H. Chen, L. Li, E.-W. Niu, H. Pang, B. Zou, and S.-Z Yang, “Investigation of plasma electrolytic oxidation process on AZ91D magnesium alloy,” Curr. Appl. Phys., 9, No. 1, 126–130 (2009).
O. Khaselev, D. Weiss, and J. Yahalom, “Structure and composition of anodic films formed on binary Mg–Al alloys in KOH–aluminate solutions under continuous sparking,” Corr. Sci., 43, 1295–1307 (2001).
Y. Ma, X. Nie, D. O. Northwood, and H. Hu, “Systematic study of the electrolytic plasma oxidation process on a Mg alloy for corrosion protection,” Thin Solid Films, 49, 296–301 (2006).
L. Wen, Y. Wang, Y. Jin, B. Liu, Yu. Zhou, and D. Sun, “Microarc oxidation of 2024 Al alloy using spraying polar and its influence on microstructure and corrosion behavior,” Surf. Coat. Technol., 228, 92–99 (2013).
M. M. Student, I. B. Ivasenko, V. M. Posuvailo, H. H. Veselivs’ka, A. Yu. Pokhmurs’kyi, Ya. Ya. Sirak, and V. M. Yus’kiv, “Influence of the porosity of a plasma-electrolytic coating on the corrosion resistance of D16 alloy,” Fiz.-Khim. Mekh. Mater., 54, No. 6, 130–137 (2018); English translation: Mater. Sci., 54, No. 6, 899–906 (2019).
M. D. Klapkiv, Determination of Physicochemical Parameters of the Process of Synthesis in Electrolytic Plasma of Oxide-Ceramic Coatings on Aluminum Alloys [in Ukrainian], Author’s Abstr. of the Candidate-Degree Thesis (Engineering), Lviv (1996).
H. V. Pokhmurs’ka, M. D. Klapkiv, V. M. Posuvailo, S. Muecklich, and I. Ozdemir, “Electrochemical properties of the PEO coatings on AZ31 magnesium alloy produced by different technologies,” Fiz.-Khim. Mekh. Mater., 51, No. 1, 102–107 (2015); English translation: Mater. Sci., 51, No. 1, 114–120 (2015).
Y. Zhang, W. Fan, H. Q. Du, and Y. W. Zhao, “Corrosion behavior and structure of plasma electrolytic oxidation coated aluminium alloy,” Int. J. Electrochem. Sci., 12, 6788–6800 (2017).
V. M. Samartsev, I. D. Zartsyn, A. P. Karavaeva, and I. K. Marshakov, “Anionic activation of aluminum in the course of its anodic dissolution in halide-containing media,” Zashch. Met., 28, No. 5, 760–767 (1992).
W. Vedder and D. A. Vermilyea, “Aluminum + water reaction,” Trans. Faraday Soc., 65, No. 554, 561–564 (1969).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 56, No. 4, pp. 105–113, July–August, 2020.
Rights and permissions
About this article
Cite this article
Student, M.М., Veselivska, H.H., Kalakhan, O.S. et al. Influence of the Conditions of Plasma-Electrolytic Treatment of D16T Aluminum Alloy on its Corrosion Resistance in 3% NaCl Solution. Mater Sci 56, 550–559 (2021). https://doi.org/10.1007/s11003-021-00463-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-021-00463-z