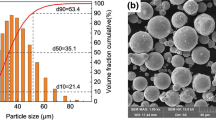
We describe an electrochemical method for the formation of composite electrochemical coatings and foils based on nickel and reinforced with nanosized aluminum oxide from a sulfamate electrolyte. We propose a mechanism of formation of composites and a mathematical model that reflects the relationship between the content of the hardening phase in composite electrochemical coatings and the concentration of Al2O3 hydrosol in the electrolyte. The influence of Al2O3 nanoparticles on the morphology and grain sizes of the composites is established by the methods of SEM and X-ray microanalysis. We determine the topography of composite electrochemical coatings and foils by the method of atomic force microscopy and establish the formation of a uniformly globular structure after the addition of Al2O3. The incorporation of nanosized particles of Al2O3 into the matrix of the base metal promotes a decrease in the grain size and the improvement of the mechanical properties of the composites: the microhardness and ultimate strength of the composites become 1.5–2 times higher and a substantial increase in the yield strength (as compared with nickel coating) is observed. The corrosion resistance of the composite electrochemical coatings increases as a result of the incorporation of aluminum oxide particles and, according to the depth index of corrosion k h = 10- 3 mm/yr we can attribute Ni–Al2O3 to the group of corrosion-resistant materials.






Similar content being viewed by others
References
L. I. Antropov and Yu. N. Lebedinskii, Composite Electrochemical Coatings and Materials [in Russian], Tekhnika, Kiev (1986).
M. Wörle, F. Krumeich, F. Bieri, H.-J. Muhr, and R. Nesper, “Flexible V7O16 layers as common structural element of vanadium oxide nanotubes,” Z. Anorg. Allg. Chem., 628, No. 12, 2778–2784 (2002).
S. V. Volkov, E. P. Koval’chuk, V. M. Ohenko, and O. V. Reshetnyak, Nanochemistry, Nanosystems, and Nanomaterials: a Monograph [in Ukrainian], Naukova Dumka, Kyiv (2008).
N. P. Lyakishev, M. I. Alymov, and V. S. Dobatkin, “Nanomaterials for structural application,” Konvers. Mashinostr., No. 6 (55), 125–130 (2002).
R. S. Saifulin, Composite Coatings and Materials [in Russian], Khimiya, Moscow (1977).
V. A. Il’in, “Nanotechnologies of deposition of cluster galvanic coatings,” Aviats. Mater. Tekhnol., No. 2, 3–6 (2009).
K. I. Portnoi, B. N. Babich, and I. L. Svetlov, Composite Nickel-Based Materials [in Russian], Metallurgiya, Moscow (1979).
М. D. Sakhnenko, О. О. Ovcharenko, M. V. Ved’, and S. І. Lyabuk, “Physicomechanical properties of Cu−Al2O3 electroplating compositions,” Fiz.-Khim. Mekh. Mater., 50, No. 5, 23–28 (2014); English translation: Mater. Sci., 50, No. 5, 646–652 (2015).
N. D. Sakhnenko, O. A. Ovcharenko, and M. V. Ved’, “Electrochemical synthesis of nickel-based composite materials modified with nanosized aluminum oxide,” Russ. J. Appl. Chem., 88, No. 2, 267−271 (2015).
N. D. Sakhnenko, M. V. Ved, Yu. K. Hapon, and T. A. Nenastina, “Functional coatings of ternary alloys of cobalt with refractory metals,” Russ. J. Appl. Chem., 88, No. 12, 1941−1945 (2015).
N. Sakhnenko, O. Ovcharenko, and M. Ved, “Copper (nickel) based composite coatings reinforced with nanosized oxides,” Funct. Mater., 22, No. 1, 105–109 (2015).
M. Castrillon, C. Garcia, and C. Paucar, “Evaluation of the influence of the particle size and the time of thermal treatment on the physical-mechanical characteristics of a composite of sintered alumina infiltrated with a lanthanum glass,” Dyna, No. 152, 159–165 (2007).
I. M. Kovenskii and V. V. Povetkin, Physical Metallurgy of Coatings [in Russian], Intermet Engineering, Moscow (1999).
M. V. Ved’, M. D. Sakhnenko, H. V. Karakurkchi, I. Yu. Ermolenko, and L. P. Fomina, “Functional properties of Fe–Mo and Fe–Mo–W galvanic alloys,” Fiz.-Khim. Mekh. Mater., 51, No. 5, 98–106 (2015); English translation: Mater. Sci., 51, No. 5, 701−710 (2016).
A. A. Bezdenezhnykh, Engineering Methods for the Formation of Equations for Reaction Rates and the Evaluation of Kinetic Constants [in Russian], Khimiya, Leningrad (1973).
О. S. Komarov, V. N. Kovalevskii, А. S. Chaus, О. V. Khrenov, B. M. Danilko, and V. Е. Chigrinov, Technology of Structural Materials [in Russian], Novoe Znanie, Minsk (2005).
M. Ghaderi, M. Rezagholizadeh, A. Heidary, and S. M. Monirvaghefi, “The effect of Al2O3 nanoparticles on the tribological and corrosion behavior of electroless Ni–B–Al2O3 composite coating,” Prot. Met. Phys. Chem. Surf., 52, No. 5, 854–858 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 53, No. 3, pp. 76–85, May–June, 2017.
Rights and permissions
About this article
Cite this article
Sakhnenko, М.D., Ved’, М.V. & Ovcharenko, О.О. Physicomechanical Properties of Composite Electrochemical Coatings and Foils Based on Nickel and Reinforced with Al2O3 . Mater Sci 53, 374–384 (2017). https://doi.org/10.1007/s11003-017-0085-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-017-0085-8