Abstract
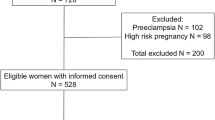
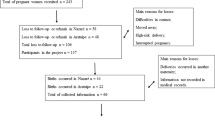
Objective The aim of the present research was to evaluate the correlation of vertically transmitted IgG antibodies induced by T. cruzi and newborn early outcome assessment, mainly birth weight and gestational age. Methods We performed a cross-sectional study with 183 pregnant women (64 with asymptomatic Chagas disease) and their newborns. Both were subjected to complete clinical examination. Peripheral parasitemia was assessed in mother and neonates by parasite detection through microscopic examination of the buffycoat from mother’s peripheral and cord blood. Antibodies induced by T. cruzi, such as anti-FRA, anti-B13, anti-p2β and anti-T. cruzi were assessed by immunoassay. Birth weight, general condition evaluation by APGAR Score and gestational age by Capurro Score, were determined in newborns. Results The rate of stillbirth background and pregnancy-induced hypertension were higher in patients with Chagas disease (p = 0.01 and p = 0.02, respectively). Parasitemia was detectable in 17 mothers and 4 newborns. The newborns of mothers with detectable parasitemia presented decreased gestational age (p = 0.006) and body weight (p = 0.04). Mostly all the mothers with Chagas disease and all their newborns have positive values of antibodies induced by T. cruzi; however, only anti-p2β showed to be related to the presence of complication during pregnancy (OR 2.35, p = 0.036), and to low birth weight (OR 1.55, p = 0.02). Conclusions Low birth weight and decreased postnatal estimation of maturity were related to detectable parasitemia in the mother. Also, vertical transmission of T. cruzi-induced autoantibodies might have clinical implication in newborns given the negative association between anti-p2β values and weight.

Similar content being viewed by others
References
Coura, J. R., & Viñas, P. A. (2010). Chagas disease: A new worldwide challenge. Nature, 465(7301), S6–S7.
Organización Panamericana de la salud. (2006). Estimación cuantitativa de la enfermedad de Chagas en las Américas. OPS/HDM/CD/425-06, Montevideo, Uruguay.
Howard, E. J., Xiong, X., Carlier, Y., Sosa-Estani, S., & Buekens, P. (2014). Frequency of the congenital transmission of Trypanosoma cruzi: A systematic review and meta-analysis. BJOG, 121(1), 22–33.
Freilij, H., & Altchech, J. (1995). Congenital Chagas disease: Diagnostic and clinical aspects. Clinical Infectious Diseases, 21, 551–555.
Sánchez Negrette, O., Mora, M. C., & Basombrío, M. A. (2005). High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics, 115(6), e668–e672.
Torrico, F., Alonso-Vega, C., Suarez, E., Rodriguez, P., et al. (2004). Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. American Journal of Tropical Medicine and Hygeine, 70(2), 201–209.
Bern, C., Martin, D. L., & Gilman, R. H. (2011). Acute and congenital Chagas disease. Advances in Parasitology, 75, 19–47.
Coura, J. R., & Borges-Pereira, J. (2010). Chagas disease: 100 years after its discovery. A systemic review. Acta Tropica, 115(1–2), 5–13.
Cunha-Neto, E., Teixeira, P. C., Nogueira, L. G., & Kalil, J. (2011). Autoimmunity. Advances in Parasitology, 76, 129–152.
Cunha-Neto, E., Coelho, V., Guilherme, L., Fiorelli, A., Stolf, N., & Kalil, J. (1996). Autoimmunity in Chagas’ disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient. Journal of Clinical Investigation, 98(8), 1709–1712.
Abel, L. C., & Kalil, J. (1997). Cunha Neto E. Molecular mimicry between cardiac myosin and Trypanosoma cruzi antigen B13: Identification of a B13-driven human T cell clone that recognizes cardiac myosin. Brazilian Journal of Medical and Biological Research, 30(11), 1305–1308.
Vicco, M. H., Ferini, F., Rodeles, L., et al. (2013). Assessment of cross-reactive host-pathogen antibodies in patients with different stages of chronic chagas disease. Revista Espanola de Cardiologia, 66(10), 791–796.
Vicco, M. H., Pujato, N., Bontempi, I., Rodeles, L., Marcipar, I., & Bottasso, O. (2014). Increased levels of anti-p2β antibodies and decreased cardiac involvement in patients with progressive chronic Chagas heart disease undergoing β1 selective antagonist treatment. Canadian Journal of Cardiology, 30(3), 332–337.
Cremaschi, G., Fernandez, M., Gorelik, G., et al. (2004). Modulatory effects on myocardial physiology induced by an anti- monoclonal antibody involve recognition of major antigenic epitopes from β-adrenergic and M-muscarinic cholinergic receptors without requiring receptor cross-linking. Journal of Neuroimmunology, 153(1–2), 99–107.
Joensen, L., Borda, E., Kohout, T., Perry, S., García, G., & Sterin-Borda, L. (2003). Trypanosoma cruzi antigen that interacts with the β1-adrenergic receptor and modifies myocardial contractile activity. Molecular and Biochemical Parasitology, 127(2), 169–177.
Labovsky, V., Smulski, C. R., Gómez, K., Levy, G., & Levin, M. J. (2007). Anti-beta1-adrenergic receptor autoantibodies in patients with chronic Chagas heart disease. Clinical and Experimental Immunology, 148(3), 440–449.
Levy, G. V., Tasso, L. M., Longhi, S. A., et al. (2011). Antibodies against the Trypanosoma cruzi ribosomal P proteins induce apoptosis in HL-1 cardiac cells. International Journal for Parasitology, 41(6), 635–644.
Robinson, D. P., & Klein, S. L. (2012). Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Hormones and Behavior, 62(3), 263–271.
Wegmann, T. G., Lin, H., Guilbert, L., & Mosmann, T. R. (1993). Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunology Today, 14(7), 353–356.
Straub, R. H. (2007). The complex role of estrogens in inflammation. Endocrine Reviews, 28(5), 521–574.
Soldan, S. S., Alvarez Retuerto, A. I., Sicotte, N. L., & Voskuhl, R. R. (2003). Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. The Journal of Immunology, 171, 6267–6274.
Palmeira, P., Quinello, C., Silveira-Lessa, A. L., Zago, C. A., & Carneiro-Sampaio, M. (2012). IgG placental transfer in healthy and pathological pregnancies. Clinical and Developmental Immunology, 2012, 985646.
Kobayashi, R., Mii, S., Nakano, T., Harada, H., & Eto, H. (2009). Neonatal lupus erythematosus in Japan: A review of the literature. Autoimmunity Reviews, 8(6), 462–466.
Aznar, C., Lopez-Bergami, P., Brandariz, S., et al. (1995). Prevalence of anti-R-13 antibodies in human Trypanosoma cruzi infection. FEMS Immunology and Medical Microbiology, 12(3–4), 231–238.
Institute of Medicine (US). (2007). Committee on understanding premature birth and assuring healthy outcomes. Measurement of fetal and infant maturity. In R. E. Behrman, A. S. Butler (Eds.), Preterm birth: Causes, consequences, and prevention.
Marcipar, S., Lagier, C. Advances in serological diagnosis of chagas’ disease by using recombinant proteins. In: A Rodriguez-Morales (Ed.), Current topics in tropical medicine. ISBN: 978-953-51-0274-8, InTech. Available from http://www.intechopen.com/books/current-topics-in-tropicalmedicine/advances-in-serological-diagnosis-of-chagas-disease-by-using-recombinant-proteins.
Freilij, H., & Altcheh, J. (1995). Congenital Chagas’ disease: Diagnostic and clinical aspects. Clinical Infectious Diseases, 21(3), 551–555.
Mora, M. C., Sanchez Negrette, O., Marco, D., et al. (2005). Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. Journal of Parasitology, 91(6), 1468–1473.
Virreira, M., Torrico, F., Truyens, C., et al. (2003). Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. American Journal of Tropical Medicine and Hygeine, 68, 574–582.
Carlier, Y., Torrico, F., Sosa-Estani, S., et al. (2011). Congenital Chagas disease: Recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Neglected Tropical Disease, 5(10), e1250. doi:10.1371/journal.pntd.0001250.
Buekens, P., Cafferata, M. L., Alger, J., et al. (2013). Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: Study protocol. Reproductive Health, 11(10), 55. doi:10.1186/1742-4755-10-55.
Bua, J., Volta, B. J., Perrone, A. E., et al. (2013). How to improve the early diagnosis of Trypanosoma cruzi infection: Relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Neglected Tropical Disease, 7(10), e2476. doi:10.1371/journal.pntd.0002476.
Oliveira, I., Torrico, F., Mũnoz, J., & Gascon, J. (2010). Congenital transmission of Chagas disease: A clinical approach. Expert Review Anti-Infect Therapy, 8, 945–956.
Carlier, Y., Truyens, C., Deloron, P., & Peyron, F. (2012). Congenital parasitic infections: A review. Acta Tropica, 121(2), 55–70.
Vicco, M. H., Bontempi, I. A., Rodeles, L., Yodice, A., Marcipar, I. S., & Bottasso, O. (2014). Decreased level of antibodies and cardiac involvement in patients with chronic Chagas heart disease vaccinated with BCG. Medical Microbiology and Immunology, 203(2), 133–139.
Marcipar, I. S., Roodveldt, C., Corradi, G., et al. (2005). Use of full-length recombinant calflagin and its c fragment for improvement of diagnosis of Trypanosoma cruzi infection. Journal of Clinical Microbiology, 43(11), 5498–5503.
Camussone, C., Gonzalez, V., Belluzo, M. S., et al. (2009). Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clinical and Vaccine Immunology, 16(6), 899–905.
Tijssen, P. (1985). Processing of data and reporting of results of enzymeimmunoassays. In: Practice and theory of enzimeimmunoassays. Laboratory techniques in biochemistry and molecular biology (Vol. 15, pp. 385–421). Amsterdam: Elsevier.
Wright, P. F., Nilsson, E., Van Rooij, E. M., Lelenta, M., & Jeggo, M. H. (1993). Standardisation and validation of enzyme-linked immunosorbent assay techniques for the detection of antibody in infectious disease diagnosis. Revue Scientifique et Technique, 12(2), 435–450.
Wahrenberg, H., Arner, P., Engfeldt, P., Haglund, K., Rössner, S., & Ostman, J. (1985). Long-term beta 1-selective adrenergic blockade and adrenergic receptors in human subcutaneous adipocytes. Acta Medica Scandinavica, 217(5), 539–546.
Hoffstedt, J., Arner, P., Hellers, G., & Lönnqvist, F. (1997). Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. Journal of Lipid Research, 38(4), 795–804.
Langin, D. (2006). Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacological Research, 53(6), 482–491.
Jaworski, K., Sarkadi-Nagy, E., Duncan, R. E., Ahmadian, M., & Sul, H. S. (2007). Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. American Journal of Physiology. Gastrointestinal and Liver Physiology, 293(1), G1–G4.
Blaak, E. E., Van Baak, M. A., Kemerink, G. J., Pakbiers, M. T., Heidendal, G. A., & Saris, W. H. (1994). Beta-Adrenergic stimulation of skeletal muscle metabolism in relation to weight reduction in obese men. American Journal of Physiology, 267(2 Pt 1), E316–E322.
Jocken, J. W., Goossens, G. H., van Hees, A. M., et al. (2008). Effect of beta-adrenergic stimulation on whole-body and abdominal subcutaneous adipose tissue lipolysis in lean and obese men. Diabetologia, 51(2), 320–327.
Gürtler, R. E., Segura, E. L., & Cohen, J. E. (2003). Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerging Infectious Diseases, 9(1), 29–32.
Fretes, R. E., & Kemmerling, U. (2012). Mechanism of Trypanosoma cruzi placenta invasion and infection: The use of human chorionic villi explants. Journal of Tropical Medicine, 2012, 614820.
Saji, F., Koyama, M., & Matsuzaki, N. (1994). Current topic: Human placental Fc receptors. Placenta, 15(5), 453–466.
Vicco, M. H., Rodeles, L., Yódice, A., Marcipar, I. (2014). Chagas disease, a risk factor for high blood pressure. Blood Pressure. [Epub ahead of print].
Johnson, J. A., & Liggett, S. B. (2011). Cardiovascular pharmacogenomics of adrenergic receptor signaling: Clinical implications and future directions. Clinical Pharmacology and Therapeutics, 89(3), 366–378.
Nguyen, G., Delarue, F., Burcklé, C., Bouzhir, L., Giller, T., & Sraer, J. D. (2002). Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. JCI, 109, 1417–1427.
Suzuki, Y., Ruiz-Ortega, M., Lorenzo, O., Ruperez, M., Esteban, V., & Egido, J. (2003). Inflammation and angiotensin II. International Journal of Biochemistry & Cell Biology, 35(6), 881–900.
Chen, C., Du, J., Feng, W., Song, Y., Lu, Z., Xu, M., et al. (2012). β-Adrenergic receptors stimulate interleukin-6 production through Epac-dependent activation of PKCδ/p38 MAPK signalling in neonatal mouse cardiac fibroblasts. British Journal of Pharmacology, 166(2), 676–688.
Lockwood, C. J., Yen, C. F., Basar, M., Kayisli, U. A., Martel, M., Buhimschi, I., et al. (2008). Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. American Journal of Pathology, 172(6), 1571–1579.
Sharma, A. M., Pischon, T., Hardt, S., Kunz, I., & Luft, F. C. (2001). Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension, 37(2), 250–254.
Acknowledgments
M.H.V L.R and M. P are research fellows of the National Scientific and Technical Research Council (CONICET). I.S.M and O.B. are research career members of CONICET. This work was in part supported by the “Institut de Recherche pour le Développement (IRD) through its program of “JeunesEquipesAssociées à l´IRD”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There was no conflicts of interests.
Additional information
Miguel Hernán Vicco and Luz Rodeles have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Vicco, M.H., Rodeles, L., Capovilla, G.S. et al. IgG Autoantibodies Induced by T. cruzi During Pregnancy: Correlation with Gravidity Complications and Early Outcome Assessment of the Newborns. Matern Child Health J 20, 2057–2064 (2016). https://doi.org/10.1007/s10995-016-2035-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-016-2035-8