Abstract
Escape from apoptosis is one of the main demeanor characters of cancer cells. Mitochondria is a key player in the initiation and regulation intrinsic apoptosis. Altering the outer mitochondrial membrane permeability can elicit cytochrome C release, which is a vital step in the intrinsic apoptosis pathway. Targeting the pore-forming protein and its regulators, known as Bcl2 family proteins, is a useful strategy to regulate apoptosis. This article provides a review of synthetic peptides that stimulate apoptosis via the mitochondrial pathways in the hopes of developing future cancer treatments. The role of mitochondria in apoptotic signaling is discussed as well as we outlined the mechanistic role of a few peptides that have been reported for cytotoxic effects, which may be useful to scientists working on therapeutic approaches. It was believed that if the extrinsic pathway failed to induce cell death, the intrinsic pathway. Apoptotic activity of peptides produced from the Bcl2 family, antimicrobial peptides, and other membrane protein-derived peptides has paved the way for the development of new generation anticancer therapeutic agents. However, if this fantastic scenario is to come true, molecular pharmacologists and peptide chemists will have to start working together rather than separately.
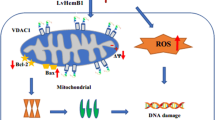
Graphic Abstract



Similar content being viewed by others
References
Alves ID, Carré M, Montero MP et al (2014) A proapoptotic peptide conjugated to penetratin selectively inhibits tumor cell growth. Biochim Biophys Acta - Biomembr 1838:2087–2098. https://doi.org/10.1016/j.bbamem.2014.04.025
Amanakis G, Murphy E (2020) Cyclophilin D: An Integrator of Mitochondrial Function. Front Physiol 11:1–6
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Bergstrom CL, Beales PA, Lv Y et al (2013) Cytochrome c causes pore formation in cardiolipin-containing membranes. Proc Natl Acad Sci USA 110:6269–6274
Beutner G, Alanzalon RE, Porter GA (2017) Cyclophilin D regulates the dynamic assembly of mitochondrial ATP synthase into synthasomes. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-14795-x
Brenner C, Subramaniam K, Pertuiset C et al (2011) Adenine nucleotide translocase family: four isoforms for apoptosis modulation in cancer. Oncogene 30:883–895
Brouwer JM, Lan P, Cowan AD et al (2017) Conversion of Bim-BH3 from activator to inhibitor of Bak through structure-based design. Mol Cell 68:659-672.e9. https://doi.org/10.1016/j.molcel.2017.11.001
Candé C, Cohen I, Daugas E et al (2002) Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84:215–222
Cao Z, Jia Y, Zhu B (2019) BNP and NT-proBNP as diagnostic biomarkers for cardiac dysfunction in both clinical and forensic medicine. Int J Mol Sci 20:18–20
Chen X, Hu C, Zhang Y et al (2020) Anticancer activity and mechanism of action of kla-TAT peptide. Int J Pept Res Ther 26:2285–2296
Chin HS, Li MX, Tan IKL et al (2018) VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat Commun. https://doi.org/10.1038/s41467-018-07309-4
Dadsena S, Bockelmann S, Mina JGM et al (2019) Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun. https://doi.org/10.1038/s41467-019-09654-4
Díaz-Quintana A, Pérez-Mejías G, Guerra-Castellano A, et al (2020) Wheel and deal in the mitochondrial inner membranes: the tale of cytochrome c and cardiolipin. Oxid Med Cell Longev
Dubey AK, Godbole A, Mathew MK (2016) Regulation of VDAC trafficking modulates cell death. Cell Death Discov 2
n, Eunsung Mouradian MM (2008) 基因的改变NIH Public Access 23:1–7
Flores-Romero H, García-Sáez AJ (2019) The incomplete puzzle of the BCL2 proteins. Cells 8
Gogvadze V, Robertson JD, Enoksson M et al (2004) Mitochondrial cytochrome c release may ocour by volume-dependent mechanisms not involving permeability transition. Biochem J 378:213–217
Green DR, Llambi F (2015) Smac mimetics as novel promising modulators of apoptosis in the treatment of breast cancer. Cold Spring Harb Perspect Biol 7
Han Q, Zhang C, Zhang Y et al (2021) Bufarenogin induces intrinsic apoptosis via Bax and ANT cooperation. Pharmacol Res Perspect 9:1–11
Hao W, Hu C, Huang Y et al (2019) Coadministration of kla peptide with HPRP-A1 to enhance anticancer activity. PLoS One 14:1–15
Hernández-luna MA, León-ortega De RD, Hernández-cueto DD et al (2016) Bactofection of sequences encoding a Bax protein peptide chemosensitizes prostate cancer tumor cells. Bol Med Hosp Infant Mex 73:388–396. https://doi.org/10.1016/j.bmhimx.2016.10.002
Hird AW, Tron AE (2019) Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol Ther 198:59–67. https://doi.org/10.1016/j.pharmthera.2019.02.007
Huang Y, Li X, Sha H et al (2017) Tumor-penetrating peptide fused to a pro-Apoptotic peptide facilitates effective gastric cancer therapy. Oncol Rep 37:2063–2070
Huang K, O’Neill KL, Li J et al (2019) BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis. Cell Res 29:942–952. https://doi.org/10.1038/s41422-019-0231-y
Kalpage HA, Wan J, Morse PT, et al (2020) Cytochrome c phosphorylation: control of mitochondrial electron transport chain flux and apoptosis. Int J Biochem Cell Biol 121
Kammath AJ, Nair B, Sreelekshmi P, et al (2021) Curry versus cancer: Potential of some selected culinary spices against cancer with in vitro, in vivo, and human trials evidences. J Food Biochem 45 https://pubmed.ncbi.nlm.nih.gov/32524639/
Karch J, Bround MJ, Khalil H, et al (2018) Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. bioRxiv 1–8
Kehr S, Haydn T, Bierbrauer A et al (2020) Targeting BCL-2 proteins in pediatric cancer: dual inhibition of BCL-XL and MCL-1 leads to rapid induction of intrinsic apoptosis. Cancer Lett 482:19–32. https://doi.org/10.1016/j.canlet.2020.02.041
Kim H, Tu HC, Ren D et al (2009) Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36:487–499. https://doi.org/10.1016/j.molcel.2009.09.030
Koushi M, Aoyama Y, Kamei Y et al (2020) Bisindolylpyrrole triggers transient mitochondrial permeability transitions to cause apoptosis in a VDAC1/2 and cyclophilin D-dependent manner via the ANT-associated pore. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-73667-z
Krebs J (1998) The role of calcium in apoptosis. Biometals 11:375–382
Kuo HM, Tseng CC, Chen NF, et al (2018) MSP-4, an antimicrobial peptide, induces apoptosis via activation of extrinsic Fas/FasL- and intrinsic mitochondria-mediated pathways in one osteosarcoma cell line. Mar Drugs 16
Kurrikoff K, Aphkhazava D, Langel Ü (2019) The future of peptides in cancer treatment. Curr Opin Pharmacol 47:27–32
Kuwana T, Bouchier-Hayes L, Chipuk JE et al (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17:525–535
Lauterwasser J, Todt F, Zerbes RM et al (2016) The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation. Sci Rep 6:1–11
Lavik AR, Zhong F, Chang MJ et al (2015) BIRD-2. Oncotarget 6:27388–27402
Li H, Zhu H, Xu CJ et al (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Li LY, Luo X, Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412:95–99
Liu G, Wang ZK, Wang ZY et al (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90:1193–1209
Lopez J, Tait SWG (2015) Mitochondrial apoptosis: Killing cancer using the enemy within. Br J Cancer 112:957–962. https://doi.org/10.1038/bjc.2015.85
Lu J, Yang J, Zheng Y et al (2019) Resveratrol reduces store-operated Ca(2+) entry and enhances the apoptosis of fibroblast-like synoviocytes in adjuvant arthritis rats model via targeting ORAI1-STIM1 complex. Biol Res 52:45
Luo X, O’Neill KL, Huang K (2020) The third model of Bax/Bak activation: a Bcl-2 family feud finally resolved? F1000Research 9: 1–15
Magrì A, Ramona B, Reina S, et al (2016) Hexokinase I N-terminal based peptide prevents the VDAC1-SOD1 G93A interaction and re-establishes ALS cell viability. Nat Publ Gr 1–14
Marini C, Salani B, Massollo M et al (2013) Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 12:3490–3499
Mateos-Chávez AA, Muñoz-López P, Becerra-Báez EI et al (2019) Live attenuated salmonella enterica expressing and releasing cell-permeable Bax BH3 peptide through the misl autotransporter system elicits antitumor activity in a murine xenograft model of human B non-hodgkin’s lymphoma. Front Immunol 10:1–22
McArthur K, Whitehead LW, Heddleston JM, et al (2018) BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359
Messina A, Reina S, Guarino F et al (2012) VDAC isoforms in mammals. Biochim Biophys Acta - Biomembr 1818:1466–1476. https://doi.org/10.1016/j.bbamem.2011.10.005
Moreno P, Ramos-Álvarez I, Moody TW et al (2016) Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin Ther Targets 20:1055–1073
Nikkhoo A, Rostami N, Hojjat-Farsangi M et al (2019) Smac mimetics as novel promising modulators of apoptosis in the treatment of breast cancer. J Cell Biochem 120:9300–9314
Peptide S, Death MC (2020) Molecules associated with cytoplasmic membrane disruption, mitochondrial dysfunction and cell cycle arrest in 2020
Pinto IFD, de Chaves-Filho A, Cunha da, D et al (2020) Cytochrome c modification and oligomerization induced by cardiolipin hydroperoxides in a membrane mimetic model. Arch Biochem Biophys 693:108568. https://doi.org/10.1016/j.abb.2020.108568
Rai Y, Yadav P, Kumari N et al (2019) Hexokinase II inhibition by 3-bromopyruvate sensitizes myeloid leukemic cells K-562 to anti-leukemic drug, daunorubicin. Biosci Rep 39:1–18
Rogers C, Erkes DA, Nardone A et al (2019) Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun 10:1–17. https://doi.org/10.1038/s41467-019-09397-2
Ruan J (2019) Structural insight of gasdermin family driving pyroptotic cell death. Adv Exp Med Biol 1172:189–205
Sasidharan A, Chandran P, Menon D et al (2011) Rapid dissolution of ZnO nanocrystals in acidic cancer microenvironment leading to preferential apoptosis. Nanoscale 3:3657–3669
Shteinfer-kuzmine A, Amsalem Z, Arif T, et al (2018) Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy. 12:1077–1103
Su B, Liu Y, Ting C, et al (2020) Antimicrobial peptide TP4 targets mitochondrial. 1–13
Tait SWG, Green DR (2010) Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11:621–632. https://doi.org/10.1038/nrm2952
Tewari D, Majumdar D, Vallabhaneni S et al (2017) Aspirin induces cell death by directly modulating mitochondrial voltage-dependent anion channel (VDAC). Sci Rep 7:1–9
Ting CH, Chen YC, Wu CJ et al (2016) Targeting FOSB with a cationic antimicrobial peptide, TP4, for treatment of triple-negative breast cancer. Oncotarget 7:40329–40347
Ting CH, Liu YC, Lyu PC et al (2018) Nile tilapia derived antimicrobial peptide TP4 exerts antineoplastic activity through microtubule disruption. Mar Drugs 16:1–16
Tse C, Shoemaker AR, Adickes J et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12:835–840
Ugarte-alvarez O, Muñoz-l P, Moreno-vargas LM, et al (2020) Cell-permeable Bak BH3 peptide induces chemosensitization of 2020
Uren RT, Iyer S, Kluck RM (2017) Pore formation by dimeric Bak and Bax: an unusual pore? Philos Trans R Soc B Biol Sci 372
Wang Z, Yin F, Xu J et al (2019) CYT997(Lexibulin) induces apoptosis and autophagy through the activation of mutually reinforced ER stress and ROS in osteosarcoma. J Exp Clin Cancer Res 38:44
Wei MC, Lindsten T, Mootha VK et al (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 14:2060–2071
Wei L, Zhou Y, Dai Q et al (2013) Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis 4:1–12
Whang J, Back YW, Lee KI et al (2017) Mycobacterium abscessus glycopeptidolipids inhibit macrophage apoptosis and bacterial spreading by targeting mitochondrial cyclophilin D. Cell Death Dis 8:e3012. https://doi.org/10.1038/cddis.2017.420
Willis SN (2008) Apoptosis initiated when BH3 ligands 856
Wu K, Luan G, Xu Y et al (2020) Cigarette smoke extract increases mitochondrial membrane permeability through activation of adenine nucleotide translocator (ANT) in lung epithelial cells. Biochem Biophys Res Commun 525:733–739. https://doi.org/10.1016/j.bbrc.2020.02.160
Xin M, Li R, Xie M, et al (2014) Small-molecule Bax agonists for cancer therapy. Nat Commun 5
Zhang M, Zheng J, Nussinov R et al (2017) Release of cytochrome C from Bax pores at the mitochondrial membrane /631/114/2397 /639/638/440/56 /119/118 article. Sci Rep 7:1–13
Zhang X, Brossas JY, Parizot C et al (2018) Identification and characterization of novel enhanced cell penetrating peptides for anti-cancer cargo delivery. Oncotarget 9:5944–5957
Zhou H, Forveille S, Sauvat A et al (2015) The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget 6:26599–26614
Acknowledgements
We gratefully acknowledge the Principal and HOD, Amrita School of Pharmacy, for their support and assistance.
Funding
The authors received no specific funding for this work. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflict of interest associated with this publication.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Since the work does not contain any studies with human participants, no consents are required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslam, M., Kanthlal, S.K. & Panonummal, R. Peptides: A Supercilious Candidate for Activating Intrinsic Apoptosis by Targeting Mitochondrial Membrane Permeability for Cancer Therapy. Int J Pept Res Ther 27, 2883–2893 (2021). https://doi.org/10.1007/s10989-021-10297-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-021-10297-7