Abstract
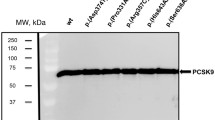
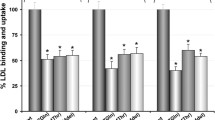
Familial hypercholesterolemia (FH) is a metabolic disease caused by the inherited pathogenic mutations in PCSK9 gene. This study has evaluated the potential of diverse computational methods in predicting the PCSK9 clinical variants and also studied the impacts of mutation on protein phenotype and function. Our findings show the superior prediction ability of FATHMM over CADD, M-CAP, SIFT and Polyphen methods, in screening PCSK9 missense mutations causative to FH. Computational 3D mapping of PCSK9 variants located in prodomain (K83T), catalytic domain (D204N) and c-terminal domains (K494E) revealed mutant residue induced disturbances in the tertiary structure of protein. These variants are also predicted to impair the free energy dynamics, hence stability of PCKS9 as per the consensual predictions made by protein structure-based prediction algorithms like SAAFEC and MAESTRO. Molecular docking assay by PIZMA algorithm showed the increased binding affinity between PCSK9 variants and LDLR molecules. Furthermore, the results from PDBsum method have also suggested changes in interfacing residues, interface area, salt bridge and ionic bond interactions confirming the findings from docking analysis. The PCSK9 functional domain mutations could increase its binding affinity with LDLR, eventually promoting LDLR degradation in lysosomes and elevate circulating cholesterol levels in body. This study supports the application of comprehensive computational assessment of FH causative PCSK9 mutations before undertaking labor intensive functional biology investigations.





Similar content being viewed by others
References
Abifadel M et al (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34:154–156. https://doi.org/10.1038/ng1161
Ajabnoor GMA et al (2018) Expanded somatic mutation spectrum of MED12 gene in uterine leiomyomas of Saudi Arabian women. Front Genet 9:552. https://doi.org/10.3389/fgene.2018.00552
Al-Abbasi FA, Mohammed K, Sadath S, Banaganapalli B, Nasser K, Shaik NA (2018) Computational protein phenotype characterization of IL10RA mutations causative to early onset inflammatory bowel disease (IBD). Front Genet 9:146. https://doi.org/10.3389/fgene.2018.00146
Alnouri F et al (2018) Novel combined variants of LDLR and LDLRAP1 genes causing severe familial hypercholesterolemia. Atherosclerosis 277:425–433. https://doi.org/10.1016/j.atherosclerosis.2018.06.878
Anderson ED, Molloy SS, Jean F, Fei H, Shimamura S, Thomas G (2002) The ordered and compartment-specfific autoproteolytic removal of the furin intramolecular chaperone is required for enzyme activation. J Biol Chem 277:12879–12890. https://doi.org/10.1074/jbc.M108740200
Benjannet S et al (2010) Effects of the prosegment and pH on the activity of PCSK9: evidence for additional processing events. J Biol Chem 285:40965–40978. https://doi.org/10.1074/jbc.M110.154815
Berberich AJ, Hegele RA (2019) The complex molecular genetics of familial hypercholesterolaemia. Nat Rev Cardiol 16:9–20. https://doi.org/10.1038/s41569-018-0052-6
Berman HM et al (2000) The Protein Data Bank. Nucleic Acids Res 28:235–242. https://doi.org/10.1093/nar/28.1.235
Bottomley MJ et al (2009) Structural and biochemical characterization of the wild type PCSK9-EGF(AB) complex and natural familial hypercholesterolemia mutants. J Biol Chem 284:1313–1323. https://doi.org/10.1074/jbc.M808363200
Bouhairie VE, Goldberg AC (2015) Familial hypercholesterolemia. Cardiol Clin 33:169–179. https://doi.org/10.1016/j.ccl.2015.01.001
Cameron J et al (2008) Characterization of novel mutations in the catalytic domain of the PCSK9 gene. J Intern Med 263:420–431. https://doi.org/10.1111/j.1365-2796.2007.01915.x
Capriotti E, Fariselli P, Casadio R (2005) I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res 33:W306–310. https://doi.org/10.1093/nar/gki375
Chen R, Tong W, Mintseris J, Li L, Weng Z (2003) ZDOCK predictions for the CAPRI challenge. Proteins 52:68–73. https://doi.org/10.1002/prot.10388
Chen CW, Lin MH, Liao CC, Chang HP, Chu YW (2020) iStable 2.0: predicting protein thermal stability changes by integrating various characteristic modules. Comput Struct Biotechnol J 18:622–630. https://doi.org/10.1016/j.csbj.2020.02.021
Cunningham D et al (2007) Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol 14:413–419. https://doi.org/10.1038/nsmb1235
Folkman L, Stantic B, Sattar A, Zhou Y (2016) EASE-MM: sequence-based prediction of mutation-induced stability changes with feature-based multiple models. J Mol Biol 428:1394–1405. https://doi.org/10.1016/j.jmb.2016.01.012
Getov I, Petukh M, Alexov E (2016) SAAFEC: predicting the effect of single point mutations on protein folding free energy using a knowledge-modified MM/PBSA approach. Int J Mol Sci 17:512. https://doi.org/10.3390/ijms17040512
Goksuluk D, Korkmaz S, Zararsiz G, Karaagaoglu AE (2016) easyROC: an interactive web-tool for ROC curve analysis using R language. Environ R J 8:213–230
Gschwend DA, Good AC, Kuntz ID (1996) Molecular docking towards drug discovery. J Mol Recognit 9:175–186. https://doi.org/10.1002/(sici)1099-1352(199603)9:2<175:Aid-jmr260>3.0.Co;2-d
Henrich S et al (2003) The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat Struct Biol 10:520–526. https://doi.org/10.1038/nsb941
Hijikata A, Tsuji T, Shionyu M, Shirai T (2017) Decoding disease-causing mechanisms of missense mutations from supramolecular structures. Sci Rep 7:8541. https://doi.org/10.1038/s41598-017-08902-1
Holla OL, Cameron J, Tveten K, Strom TB, Berge KE, Laerdahl JK, Leren TP (2011) Role of the C-terminal domain of PCSK9 in degradation of the LDL receptors. J Lipid Res 52:1787–1794. https://doi.org/10.1194/jlr.M018093
Holyoak T, Wilson MA, Fenn TD, Kettner CA, Petsko GA, Fuller RS, Ringe D (2003) 2.4 A resolution crystal structure of the prototypical hormone-processing protease Kex2 in complex with an Ala-Lys-Arg boronic acid inhibitor. Biochemistry 42:6709–6718. https://doi.org/10.1021/bi034434t
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30. https://doi.org/10.1093/nar/28.1.27
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–d361. https://doi.org/10.1093/nar/gkw1092
Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M (2019) New approach for understanding genome variations in KEGG. Nucleic Acids Res 47:D590–d595. https://doi.org/10.1093/nar/gky962
Kaya E, Kayikcioglu M, Tetik Vardarli A, Eroglu Z, Payzin S, Can L (2017) PCSK 9 gain-of-function mutations (R496W and D374Y) and clinical cardiovascular characteristics in a cohort of Turkish patients with familial hypercholesterolemia. Anatol J Cardiol 18:266–272. https://doi.org/10.14744/AnatolJCardiol.2017.7654
Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J (2008) Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci USA 105:1820–1825. https://doi.org/10.1073/pnas.0712064105
Laimer J, Hofer H, Fritz M, Wegenkittl S, Lackner P (2015) MAESTRO–multi agent stability prediction upon point mutations. BMC Bioinform 16:116. https://doi.org/10.1186/s12859-015-0548-6
Land H, Humble MS (2018) YASARA: a tool to obtain structural guidance in biocatalytic investigations. Methods Mol Biol 1685:43–67. https://doi.org/10.1007/978-1-4939-7366-8_4
Laskowski RA, Jablonska J, Pravda L, Varekova RS, Thornton JM (2018) PDBsum: structural summaries of PDB entries. Protein Sci 27:129–134. https://doi.org/10.1002/pro.3289
Lee C et al (2019) Effects of familial hypercholesterolemia-associated genes on the phenotype of premature myocardial infarction. Lipids Health Dis 18:95. https://doi.org/10.1186/s12944-019-1042-3
Lo Surdo P et al (2011) Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep 12:1300–1305. https://doi.org/10.1038/embor.2011.205
Martin WR, Lightstone FC, Cheng F (2020) In silico insights into protein–protein interaction disruptive mutations in the PCSK9–LDLR complex. Int J Mol Sci. https://doi.org/10.3390/ijms21051550
McLaren WGL, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The ensembl variant effect predictor. Genome Biol 1:17. https://doi.org/10.1186/s13059-016-0974-4
Morad FA et al (2018) Silico approach to investigate the structural and functional attributes of familial hypercholesterolemia variants reported in the Saudi population. J Comput Biol 25:170–181. https://doi.org/10.1089/cmb.2017.0018
Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874. https://doi.org/10.1101/gr.176601
Pierce BG, Wiehe K, Hwang H, Kim BH, Vreven T, Weng Z (2014) ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 30:1771–1773. https://doi.org/10.1093/bioinformatics/btu097
Piper DE et al (2007) The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure 15:545–552. https://doi.org/10.1016/j.str.2007.04.004
Pires DE, Ascher DB, Blundell TL (2014) mCSM: predicting the effects of mutations in proteins using graph-based signatures. Bioinformatics 30:335–342. https://doi.org/10.1093/bioinformatics/btt691
Richards S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Roy AA, Dhawanjewar AS, Sharma P, Singh G, Madhusudhan MS (2019) Protein Interaction Z Score Assessment (PIZSA): an empirical scoring scheme for evaluation of protein-protein interactions. Nucleic Acids Res 47:W331–W337. https://doi.org/10.1093/nar/gkz368
Sabir JSM et al (2019) The genetic association study of TP53 polymorphisms in Saudi obese patients Saudi. J Biol Sci 26:1338–1343. https://doi.org/10.1016/j.sjbs.2019.04.006
Sadowski CE et al (2017) BRCA1/2 missense mutations and the value of in-silico analyses. Eur J Med Genet 60:572–577. https://doi.org/10.1016/j.ejmg.2017.08.005
Sala D et al (2019) Protein structure prediction assisted with sparse NMR data in CASP13. Proteins 87:1315–1332. https://doi.org/10.1002/prot.25837
Santini S, Bizzarri AR, Cannistraro S (2011) Modelling the interaction between the p53 DNA-binding domain and the p28 peptide fragment of Azurin. J Mol Recogn 24:1043–1055. https://doi.org/10.1002/jmr.1153
Santos RD (2019) Screening and management of familial hypercholesterolemia. Curr Opin Cardiol 34:526–530. https://doi.org/10.1097/HCO.0000000000000660
Seidah NG et al (2003) The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA 100:928–933. https://doi.org/10.1073/pnas.0335507100
Shaik NA, Nasser KK, Alruwaili MM, Alallasi SR, Elango R, Banaganapalli B (2020) Molecular modelling and dynamic simulations of sequestosome 1 (SQSTM1) missense mutations linked to Paget disease of bone. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1758212
Shaik NA et al (2020) Molecular insights into the coding region mutations of low-density lipoprotein receptor adaptor protein 1 (LDLRAP1) linked to familial hypercholesterolemia. J Gene Med 22:e3176. https://doi.org/10.1002/jgm.3176
Shihab HA, Gough J, Mort M, Cooper DN, Day IN, Gaunt TR (2014) Ranking non-synonymous single nucleotide polymorphisms based on disease concepts. Hum Genomics 8:11. https://doi.org/10.1186/1479-7364-8-11
Sian Ellard ELB, Ian Berry , Natalie Forrester , Clare Turnbull , Martina Owens , Diana M Eccles , Stephen Abbs , Richard Scott, Zandra C Deans, Tracy Lester , Jo Campbell, William G Newman and Dominic J McMullan. (2019) ACGS Best Practice Guidelines for Variant Classification 2019 https://www.leedsth.nhs.uk/assets/Genetics-Laboratory/86fa75f316/ACGS-variant-classification-guidelines-2019.pdf.
Taghizadeh E et al (2019) Familial combined hyperlipidemia: an overview of the underlying molecular mechanisms and therapeutic strategies. IUBMB Life 71:1221–1229. https://doi.org/10.1002/iub.2073
Trinder M et al (2019) Risk of premature atherosclerotic disease in patients with monogenic versus polygenic familial hypercholesterolemia. J Am Coll Cardiol 74:512–522. https://doi.org/10.1016/j.jacc.2019.05.043
Warden BA, Fazio S, Shapiro MD (2019) The PCSK9 revolution: Current status, controversies, and future directions. Trends Cardiovasc Med. https://doi.org/10.1016/j.tcm.2019.05.007
Worth CL, Preissner R, Blundell TL (2011) SDM–a server for predicting effects of mutations on protein stability and malfunction. Nucleic Acids Res 39:W215–222. https://doi.org/10.1093/nar/gkr363
Yang J, Zhang Y (2015) Protein structure and function prediction using I-TASSER. Curr Protoc Bioinformatics 52:1–15. https://doi.org/10.1002/0471250953.bi0508s52
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, under Grant No. G1378-140-1440. The authors, therefore, acknowledge the DSR for technical and financial support.
Author information
Authors and Affiliations
Contributions
ZA, NS, and BB: conceptualization; BB and RB: data curation; BB: formal Analysis; ZA: funding acquisition; RB and BB; methodology; BB: Resources; BB: software; ZA, NS and BB: supervision; ON, HK, MA and BB: validation; BB: visualization; ZA, NS, BB: writing original draft; ZA, NS, RB, ON, HK, MA and BB: writing review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Awan, Z.A., Bahattab, R., Kutbi, H.I. et al. Structural and Molecular Interaction Studies on Familial Hypercholesterolemia Causative PCSK9 Functional Domain Mutations Reveals Binding Affinity Alterations with LDLR. Int J Pept Res Ther 27, 719–733 (2021). https://doi.org/10.1007/s10989-020-10121-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10121-8