Abstract
Two new somatostatin analogs with a characteristic part of the sequence in their structures, -c(Cys-Phe-Trp-Lys-Thr-Cys)-, were synthesized and analyzed in terms of their coordination abilities with copper(II) ions. Cyclic peptides analyzed in our previous work had histidine and aspartic acid moieties in their structures which were responsible for metal ion coordination. In analyzed molecules these amino acids are also present. Peptides Ac[D1,2,9,10]c(SST) and Ac[H1,2,9,10]c(SST) have four aspartic acid or four histidine moieties, respectively. Both peptides bind Cu(II) effectively. Due to similar structures and the possibility of comparing the obtained results with these for the two previously published, the coordination abilities of two new ligands are possible to propose. Moreover, the effectiveness of copper(II) ion binding by four cyclic His/Asp-analogues of somatostatin is also discussed here. The peptide with four histidine moieties is the most efficient among described ligands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatostatin (SST) is a peptide hormone naturally occurring in human body that plays a role in many physiological functions, e.g. in secretion of the human growth hormone by the pituitary gland and inhibition of insulin secretion (Dasgupta 2004; Patel 1999). Since the overexpression of somatostatin receptors has been found in cancer cells of the neuroendocrine tumors, the intensive studies on an application of somatostatin in peptide receptor radionuclide therapy (PRRT) has been started. Due to the short half-life time of the somatostatin in the physiological conditions, its analogues are used in the PRRT. Pharmaceuticals used in PRRP consist of three parts: a somatostatin analogue responsible for receptor binding, a linker and a radionuclide complex (Teunissen et al. 2005).
In our work we focused on cyclopeptides having two binding sites: a characteristic sequence with disulfide bridge between cysteinyl moieties -c(Cys-Phe-Trp-Lys-Thr-Cys)-, which is responsible for the interaction with somatostatin receptors in natural hormone (Patel 1999), and a binding site for a metal ion. This way of designing of somatostatin analogues is an innovative approach. Metal ion could be connected directly to the peptide molecule and it would allow to eliminate a linker and a chelator from precursors of somatostatin analogues potentially useful in diagnosis and treatment of cancer. The sequence of the somatostatin analogues responsible for metal binding consists of His and Asp amino acid residues, which are crucial for metal ion binding in biological systems (Cao et al. 2017; Kozłowski et al. 1999) and are good ligands for copper(II) ions (Sóvágó et al. 2012; Sóvágó and Osz 2006).
Copper(II) is a microelement playing a significant role in living systems, e.g. as ingredient of a variety of different enzymes centers, in redox reactions in essential metabolic processes (Linder 1991; Peña et al. 1999; Tapiero et al. 2003). Moreover, it is well documented that Cu(II) and its isotopes are promising tools for PRRT. Current applications of somatostatin analogues with copper radionuclides and the possibility of further application of these conjugates were described in our review (Marciniak and Brasuń 2017). 27 radioisotopes of copper are known, but only six of them are useful in nuclear medicine: 60Cu, 61Cu, 62Cu, 64Cu for diagnosis, and 64Cu, 66Cu, 67Cu in radiotherapy. The well-known coordination chemistry of Cu is helpful in designing of new radiopharmaceuticals with these radioactive isotopes. Lots of results described in the literature are very promising but there is still no pharmaceutical with copper radioisotope and somatostatin analogues acceptable in human diagnosis and treatment. However, it is worth to look for new possibilities of connection of copper radionuclides with precursors of pharmaceuticals because their potential in nuclear medicine is extensive (Wadas et al. 2007; Marciniak and Brasuń 2017).
In our previous work we presented results obtained for two somatostatin analogues consisting of two His and two Asp residues in the metal binding site but with different positions in the peptide chain (Marciniak et al. 2017). It has been found that location of His residues close to the N-terminal end of the peptide chain can significantly increase the efficacy of the metal ion coordination. Obtained results showed that slight differences in the structure of peptides significantly affect efficiency of metal ion binding. Therefore, we decided to investigate other systems with cyclic somatostatin analogues and copper(II) ions with modifications within amino acids moieties responsible for metal ion coordination.
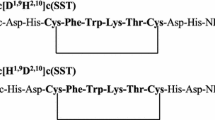
In this manuscript we present studies of two new cyclic somatostatin analogues (Fig. 1) having either 4 His or 4 Asp amino acid residues in the metal binding site. The results obtained from these studies will allow to characterize the rules responsible for metal binding by investigated peptides. The knowledge of these rules is necessary for creation of new precursors of pharmaceuticals for peptide receptor radionuclide therapy.
The visualization of the structure of somatostatin analogues: Ac-Asp-Asp-c(Cys-Phe-Trp-Lys-Thr-Cys)-Asp–Asp-NH2—Ac[D1,2,9,10]c(SST), and Ac-His-His-c(Cys-Phe-Trp-Lys-Thr-Cys)-His–His-NH2—Ac[H1,2,9,10]c(SST), prepared by ACD/Labs 2018.1.1 software. The hydrogen atoms have been removed for clarity of the image; color legend: Asp or His side chains—orange, carbon—cyan, nitrogen—blue, oxygen—red, sulfur—yellow (Color figure online)
Materials and Methods
Synthesis of the Peptides
Peptides were synthesized by the standard manual Fmoc solid-phase peptide synthesis method on the Fmoc-Rink Amide MBHA resin (0.65 mM/g, Iris Biotech GmbH). Synthesis was carried out in single-use plastic reactors (Intavis GmBH). Functional groups in the side chains of the amino acids used for the synthesis were protected as follows: Asp(OtBu), Cys(Acm), His(Trt), Lys(Boc), Thr(tBu), Trp(Boc). Subsequent Fmoc-protected amino acids (3 eq) were attached by using 3 eq PyBOP (1H-benzotriazol-1-yloxy)(tri-1-pyrrolidinyl)phosphonium hexafluorophosphate as a coupling reagent in the presence of N-hydroxybenzotriazole (3 eq) and diisopropylethylamine (6 eq) for 2 h at room temperature. Fmoc protecting groups were removed by 25% piperidine in dimethylformamide. Acetylation of the N-terminal amino group was performed on the resin by 1:1 mixture of acetic anhydride and 0.4 M N-methylmorpholine in dimethylformamide. Final cleavage of the peptides was achieved by, Reagent K” (81.5% trifluoroacetic acid, 5% phenol, 5% thioanisole, 5% water, 2.5% ethanedithiol, 1% triisopropylsilane) in 2 h at room temperature. Crude peptides, with Cys residues still protected by Acm groups, were precipitated by cold diethylether, washed with ether, dissolved in water and lyophilized.
In order to remove Acm protecting groups from the side chains of Cys residues and to form a disulfide bridge, peptides were dissolved in 80% acetic acid (2 mL of acetic acid for 2 mg of a peptide) and 20 eq of I2 dissolved in a small volume of 80% acetic acid was added. The reaction was performed under nitrogen and its progress was monitored by ESI mass spectrometry until no more unoxidized peptide was observed. Mixture was diluted with the same amount of water and excess of I2 was extracted using CCl4. Water fraction containing peptide was lyophilized.
Peptides were purified by semipreparative HPLC using Varian ProStar apparatus equipped with TOSOH Bioscience C18 column (300 Å, 21.5 mm, 10 μm beads) and 220 nm UV detector.
Water–acetonitrile gradients containing 0.1% TFA at a flow rate of 7 mL/min. were used for the purifications. Final purity of the lyophilized peptides was > 95% by analytical HPLC (Thermo Separation Product; column: Vydac Protein RP C18, 250 Å, 4.6 mm, 5 μm; linear gradient 0–100% B in 60 min., solvent A—0.1% TFA in water, solvent B—0.1% TFA in 80% acetonitrile:water solution, UV detection at 220 nm). Chemical identity of the ligands was confirmed by ESI–MS on a Bruker micrOTOF-Q or Bruker apex ultra mass spectrometer. Analytical data of the synthesized peptides are given in Table 1.
Potentiometric Measurements
Potentiometric measurements were carried out using Molspin pH-meter system with Mettler Toledo InLab 422 semimicro combined electrode at 25 °C calibrated in hydrogen ion concentration using HCl (Irving et al. 1967). The ligands concentration was 8 × 10−4 mol/L and pH-metric titrations were performed in 0.1 mol/L KCl solution using sample volumes of 1.5 mL. Alkali (NaOH) was added by using a 0.25 mL micrometer syringe. The concentration of NaOH was 0.1 mol/L. Stability constants and stoichiometry of the complexes were calculated from titration curves using the SUPERQUAD program (Gans et al. 1985). The pH range where precipitation was observed was omitted during the calculations. Due to this fact, some stability constants were only estimated, which is indicated in the “Results and Discussion” section.
Spectroscopic Measurements
Visible spectra of complexes were recorded at 25 °C on Varian Cary 50 Bio spectrophotometer. The electron paramagnetic resonance (EPR) spectra were recorded on Bruker ELEXSYS E500 CW-EPR, X-Band spectrometer, equipped with ER 036TM NMR Teslameter and E 41 FC frequency counter. Circular dichroism (CD) spectra were recorded on Jasco J-1500 magnetic circular dichroism spectrometer in 230–800 nm range. The same concentrations were used for both spectroscopic and potentiometric studies.
Results and Discussion
The acid–base properties of the studied somatostatin analogues are presented in Table 2. Both ligands are characterized by five protonation constants related to: four for aspartic acid and one for lysine moieties for Ac[D1,2,9,10]c(SST) (Table 2) and four for histidine and one for lysine moieties for Ac[H1,2,9,10]c(SST) (Table 4), and they are comparable to those found in the literature (Holm et al. 1996).
During the analysis of coordination abilities of peptides and copper(II) ions by potentiometry, in some range of pH, the precipitation was observed (areas marked in gray on Fig. 2a,b). The probable reason of this effect was an appearance of complex forms with neutral charge in the analyzed systems (Hashempour et al. 2010). Therefore, the stabilities constants have been determinated only in the pH area with no precipitation.
In the system with the somatostatin analog Ac[D1,2,9,10]c(SST), the precipitation is observed between pH 6 and 8.5. Below pH 6 two complexes, CuH2L and CuHL, exist in the system (Fig. 2a). The formation of CuH2L and CuHL species is related to the dissociation of three and four protons from the ligand molecule, respectively. Due to the fact that in the discussed conditions, beside of formed complexes, unbound copper(II) ions exist (no less than 40%, Fig. 2a) it was not possible to obtain the spectroscopic parameters for these forms by using of the available methods. Nevertheless, the values of corrected logβ*logβCuHnL–logβHnL are relatively low (= 2.38CuH2L and = 3.42CuHL) and may support the involvement of only side chain of carboxylate group in the coordination of the metal ion (Galey et al. 1991; Kállay et al. 2005).
Above the pH 8.5 no precipitation has been observed. In these conditions the CuH-2L appears in the system and achieves its highest concentration at pH 9 (Fig. 2a). The presence of the positive CT band on the CD spectrum near 325 nm (Table 3) demonstrates the involvement of amide nitrogen donors in the coordination sphere of the metal ion. The stability constant of CuH-2L, corrected with the Lys group (= − 17.89), strongly supports the presence of three amide donors in the coordination sphere of the copper ion (Kozłowski et al. 1999). The presented assumption is supported by the UV–Vis and EPR results: location of the λmax = 580 nm for d–d band in UV–Vis spectra (Table 3) and the EPR parameters (A| = 191 G, g| = 2.223, Table 4, Fig. 3a) (Peisach and Blumberg 1974).
With the increase of pH, another complex—CuH-3L exists in the system (Fig. 2a). However, its appearance does not influence significantly the spectroscopic parameters (Tables 3, 4). The logK value of the formation of this complex (9.83, Table 2) suggests proton dissociation from Lys residues, what does not influence the coordination mode of the metal ion. The appearance of the last comprehensive form, CuH-4L, is quite surprising and unusual, but there is only one explanation. The constant logKCuH-3L–CuH-4L = 11.07 corresponds to the process of hydrolysis of water coordinated to the metal ion and the creation of hydroxo complex with an increasing negative charge of species which was observed earlier in different systems described in literature (Kállay et al. 2005; Myari et al. 2001).
Afterwards, the peptide Ac[H1,2,9,10]c(SST) with four histidine moieties in its structure has been investigated (Fig. 1b). Due to the precipitation, two ranges of pH have been discussed: below pH 6.5 and above pH 9.5. Similar situation was observed in the previously analyzed system.
In the first range of pH (below pH 6.5) three complexes are formed in the system: CuH3L, CuH2L and CuHL (Fig. 2b). The ligand starts copper(II) binding at pH 3.5 by formation of the CuH3L species. The stoichiometry of this form shows dissociation of two protons from the peptide molecule. The corrected logβ* = 5.27 is comparable to logβ* = 5.75 of the complex with {2NIm} coordination mode calculated for the cyclopeptide c(HisLysHisGlyProGlyHisLysHisGlyProGly) (Kotynia et al. 2014). The formation of the second CuH2L complex is associated with proton dissociation from the third His residue, what is supported by logK value (Table 2). Furthermore, logβ* = 6.53 is similar to logβ* = 6.76 for CuH2L complex with {3NIm} coordination mode for Ac-HisHisProPheTrpLysThrPheProHisHis-NH2—SST analogue with four His residues (Marciniak et al. 2014). Therefore, it seems to be a probable way of the metal ion coordination in the analyzed peptide. Unfortunately, due to the presence of different copper(II) complexes it was not possible to obtain the spectroscopic parameters for both forms.
Above pH 5.5, the third species appears in the solution and achieves its highest concentration at pH 6 (Fig. 2b). Once again, the value of logβ* = 8.09 is similar to analogous complexes in different peptides with four histidine moieties, which supports involvement of four imidazole donors in Cu(II) binding (Kotynia et al. 2012; Marciniak et al. 2014). The location of the λmax of d–d band at 595 nm (Table 3) is comparable with the theoretical λmax = 585 nm calculated for complex with {4NIm} coordination mode (Prenesti et al. 1999). The difference of 10 nm between these two values is probably caused by the deformation of the complex from the square planar structure. Moreover, the presence of positive bands on CD spectrum (Table 3) also demonstrates the involvement of imidazole nitrogen atoms in metal ion binding.
Afterwards, the results obtained at pH above 9.5 have been analyzed and in this range of pH another two complexes are formed: CuH-2L and CuH-3L (Fig. 2b). The first one dominates in the system in pH 9.5. Precipitation is still observed here, but the value of the stability constant estimated from potentiometric results suggests the involvement of next amide nitrogen in the metal ion coordination (Table 2). The creation of the last complex, CuH-3L, is probably related to proton dissociation from Lys residue which does not take part in the coordination of Cu(II) ion. This is evidenced by logKCuH-2L–CuH-3L = 10.25 (Table 2). Obtained spectroscopic parameters confirm here metal ion coordination by four nitrogen atoms (Tables 3, 4). Experimental λmax = 530 nm on the UV–Vis spectrum is similar to the theoretical λmax = 523 nm calculated for {NIm, 3 N−} coordination mode (Prenesti et al. 1999). EPR and CD parameters are also in accordance with this way of copper(II) ion binding (Fig. 3b, Tables 3, 4).
Both peptides discussed above form the final complex with three amide donors in the coordination sphere of copper(II) ion. There are two possibilities of creating of this species: the involvement of amide donors from the N-terminal or C-terminal backbone (Fig. 4). Moreover, two other options are possible to form the 3 × Namide complex: coordination of one or two amides from the cyclic motif of the peptide chain (Fig. 4).
The replacement of Asp residues by His influences the interaction of the metal ion with amide donors from the peptide chain. Both peptides form the final complexes with three amide donors involved in the metal ion binding. The Ac[H1,2,9,10]c(SST) prefers binding of the Cu(II) by imidazole nitrogen atoms and formation of the species with the {4 × NIm} binding mode. Owing to this fact, involvement of the amide donors is observed in more basic conditions in comparison to the peptide Ac[D1,2,9,10]c(SST) (Fig. 5).
The comparison of the efficacy in metal binding between different ligands may be presented as a competition diagram, where the percentage of metal ions bounded by analyzed ligands is showed.
Aspartic acid residue is a weaker ligand for copper (II) ions than histidine moieties (Sóvágó et al. 2012)(Sóvágó and Osz 2006). Due to this fact, it can be expected that Ac[D1,2,9,10]c(SST) would be a less effective ligand for Cu(II) binding than other somatostatin analogs. The replacement of the subsequent Asp moieties influences the binding abilities and makes Ac[H1,2,9,10]c(SST) the most effective in Cu(II) binding among analyzed peptides (Fig. 6a).
Surprisingly, the cyclic structure of the ligand has no impact on copper(II) binding and the Ac[H1,2,9,10]c(SST) and its linear analogue (Marciniak et al. 2014) bind copper(II) with comparable efficiency (Fig. 6b) in contrast to most cyclopeptides (Kotynia et al. 2018).
As it was mentioned above, the insertion of the His residue influences the efficiency in Cu(II) binding. However, it influences also formation of insoluble complexes as well as extends a pH range in which these species dominate. The CuL species is insoluble for His/Asp peptides whilst next two ligands form insoluble CuH-1L complex. Figure 7 shows the tendency of formation of the precipitated complexes as a function of pH (a) and the percentage of the precipitated complex at pH 7.4 (b). The Ac[D1,2,9,10]c(SST) is a less effective ligand for copper(II) ion. It forms the insoluble species dominant in the most wide range of pH (Fig. 7a) which achieves the highest percentage of precipitation (Fig. 7b). Ac[D1,9H2,10]c(SST) is also not an effective ligand for copper(II) ions. Nevertheless, due to the presence of two His residues, the insoluble complex dominates in a more narrow range of pH and the percentage of precipitated species is also significantly lower. Interesting results can be seen in the comparison between two most effective ligands: Ac[H1,9D2,10]c(SST) and Ac[H1,2,9,10]c(SST). The peptide with four His moieties is the most effective in Cu (II) coordination. Moreover, the precipitation of the CuH-1L is observed in more basic conditions in comparison to the Ac[H1,9D2,10]c(SST). It is curious that in the physiological range of pH both form the same percentage of the insoluble species (ca. 40%).
Conclusion
In this paper we have shown that two new cyclic somatostatin analogues, with the characteristic part of the sequence: -c(Cys-Phe-Trp-Lys-Thr-Cys)-, and with four amino acids which could bind copper(II) ions: aspartic acid residues in the first ligand and histidine moieties in the second one, are effective in metal ion coordination. Their efficiency and the manners of copper(II) ions binding were compared here with somatostatin analogues described previously. The cyclic peptide Ac[H1,2,9,10]c(SST), with four histidine residues in its structure, turned out to be the most effective ligand among those analyzed so far. Obtained information is a valuable contribution to the further development of new functional chelators. What is surprising, this ligand has a similar efficiency to the linear somatostatin analogue Ac[H1,2,10,11]SST, also with four histidine moieties, described in our previous work. The cyclic structure does not increase the effectiveness of metal ion binding. In most cyclopeptides the opposite situation was observed. All cyclic octreotide analogues show interesting binding abilities toward copper(II) complexes, however all of them form insoluble complexes. Ac[H1,2,9,10]c(SST) is the most effective in copper (II) binding, however its application as a drug is not possible due to the precipitation in the physiological range of pH. Nevertheless, as it was presented above, the tendency to precipitate may be modified by e.g. insertion of additional groups influencing the charge of the complexes.
References
Cao X, Hu X, Zhang X et al (2017) Identification of metal ion binding sites based on amino acid sequences. PLoS ONE 12:1–16. https://doi.org/10.1371/journal.pone.0183756
Dasgupta P (2004) Somatostatin analogues: multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol Ther 102:61–85. https://doi.org/10.1016/j.pharmthera.2004.02.002
Galey J-F, Reverend BD-L, Lebkiri A et al (1991) Specific interactions of the β-carboxylate group of the aspartic acid residue in oligopeptides containing one, two or three such residues with copper(II) ions. A potentiometric and spectroscopic study. J Chem Soc, Dalton Trans 9:2281–2287. https://doi.org/10.1039/dt9910002281
Gans P, Sabatini A, Vacca A (1985) SUPERQUAD: an improved general program for computation of formation constants from potentiometric data. J Chem Soc, Dalton Trans 6:1195–1200. https://doi.org/10.1039/dt9850001195
Hashempour M, Razavizadeh H, Rezaie HR et al (2010) Chemical mechanism of precipitate formation and pH effect on the morphology and thermochemical co-precipitation of W-Cu nanocomposite powders. Mater Chem Phys 123:83–90. https://doi.org/10.1016/j.matchemphys.2009.12.029
Holm RH, Kennepohl P, Solomon EI (1996) Structural and functional aspects of metal sites in biology. Chem Rev 96:2239–2314. https://doi.org/10.1021/cr9500390
Irving HM, Miles MG, Pettit LD (1967) A study of some problems in determining the stoicheiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal Chim Acta 38:475–488. https://doi.org/10.1016/s0003-2670(01)80616-4
Kállay C, Várnagy K, Micera G et al (2005) Copper(II) complexes of oligopeptides containing aspartyl and glutamyl residues. Potentiometric and spectroscopic studies. J Inorg Biochem 99:1514–1525. https://doi.org/10.1016/j.jinorgbio.2005.04.009
Kotynia A, Bielińska S, Kamysz W, Brasuń J (2012) The coordination abilities of the multiHis-cyclopeptide with two metal-binding centers—Potentiometric and spectroscopic investigation. Dalton Trans 41:12114–12120. https://doi.org/10.1039/c2dt31224g
Kotynia A, Czyznikowska Z, Bielińska S et al (2014) The impact of two -GlyProGly-motifs on formation of di-copper complexes by His4-cyclopeptides. New J Chem 38:5198–5206. https://doi.org/10.1039/c4nj00689e
Kotynia A, Pap JS, Brasun J (2018) The binding abilities of homodetic cyclic His-peptides toward copper ions. Inorganica Chim Acta 472:3–11. https://doi.org/10.1016/j.ica.2017.07.028
Kozłowski H, Bal W, Dyba M, Kowalik-Jankowska T (1999) Specific structure-stability relations in metallopeptides. Coord Chem Rev 184:319–346. https://doi.org/10.1016/s0010-8545(98)00261-6
Linder MC (1991) Biochemistry of copper. Springer, Boston
Marciniak A, Brasuń J (2017) Somatostatin analogues labeled with copper radioisotopes: current status. J Radioanal Nucl Chem 313:279–289. https://doi.org/10.1007/s10967-017-5323-x
Marciniak A, Czyznikowska Z, Cebrat M et al (2014) Structural aspects of copper(II) binding by a multi-His analogue of somatostatin. Inorganica Chim Acta 416:57–62. https://doi.org/10.1016/j.ica.2014.03.020
Marciniak A, Cebrat M, Brasuń J (2017) The coordination abilities of new cyclic analogs of somatostatin. Int J Pept Res Ther 23:135–143. https://doi.org/10.1007/s10989-016-9546-4
Myari A, Malandrinos G, Deligiannakis Y et al (2001) Interaction of Cu2 + with His–Val–His and of Zn2 + with His–Val–Gly–Asp, two peptides surrounding metal ions in Cu, Zn-superoxide dismutase enzyme. J Inorg Biochem 85:253–261. https://doi.org/10.1016/s0162-0134(01)00204-5
Patel YC (1999) Somatostatin and its receptor. Introduction. Front Neuroendocrinol 20:157–198
Peisach J, Blumberg WE (1974) Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys 165:691–708. https://doi.org/10.1016/0003-9861(74)90298-7
Peña MM, Lee J, Thiele DJ (1999) A delicate balance: homeostatic control of copper uptake and distribution. J Nutr 129:1251–1260. https://doi.org/10.1093/jn/129.7.1251
Prenesti E, Daniele PG, Prencipe M, Ostacoli G (1999) Spectrum-structure correlation for visible absorption spectra of copper(II) complexes in aqueous solution. Polyhedron 18:3233–3241. https://doi.org/10.1016/s0277-5387(99)00279-x
Sóvágó I, Osz K (2006) Metal ion selectivity of oligopeptides. Dalton Trans 32:3841–3854. https://doi.org/10.1039/b607515k
Sóvágó I, Kállay C, Várnagy K (2012) Peptides as complexing agents: factors influencing the structure and thermodynamic stability of peptide complexes. Coord Chem Rev 256:2225–2233. https://doi.org/10.1016/j.ccr.2012.02.026
Tapiero H, Townsend DM, Tew KD (2003) Trace elements in human physiology and pathology. Copper Biomed Pharmacother 57:386–398. https://doi.org/10.1016/s0753-3322(03)00012-x
Teunissen JJM, Kwekkeboom DJ, de Jong M et al (2005) Peptide receptor radionuclide therapy. Best Pract Res Clin Gastroenterol 19:595–616. https://doi.org/10.1016/j.bpg.2005.04.001
Wadas TJ, Wong EH, Weisman GR, Anderson CJ (2007) Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr Pharm Des 13:3–16. https://doi.org/10.2174/138161207779313768
Acknowledgements
Work supported by Wroclaw Medical University Grant ST.D080.16.006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Marciniak, A., Kotynia, A., Cebrat, M. et al. The Analysis of the Structural Aspects of Cu(II) Binding by Cyclic His/Asp-Analogues of Somatostatin. Int J Pept Res Ther 26, 969–977 (2020). https://doi.org/10.1007/s10989-019-09900-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09900-9