Abstract
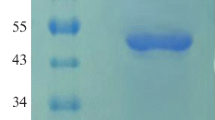
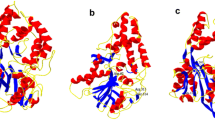
Phytase is an important enzyme poses great nutritional significance in humans and monogastric animals diets. The phytase production yield using wild sources, including micro-organisms, plants, and animals is sorely low. Thus, recombinant expression of phytase has received increasing interest for achieving production rate. Escherichia coli is the most preferred host for expression of heterologous proteins but overexpression of recombinant phytase in E. coli, met with limited success due to the sequestration of the enzyme into inclusion bodies. In the present study, artificial phytases gene with excellent thermostability and activity were designed by detecting the enzymatic region of the E. coli phytase gene by employing bioinformatics tools. Then, the PCR amplified recombinant gene was expressed in E. coli and the active enzyme was recovered from inclusion bodies. Employing cysteine amino acid in the dialysis buffer succeed to the superior activity of the enzyme with a specific activity of 73.8 U/mg. The optimum temperature and pH for enzyme activity were determined at 60 °C and 4, respectively. The novel recombinant enzyme illustrated perfect thermostability up to 70 °C with maintenance 75% of its activity. The enzyme was stable at pH range of 2–10. Moreover, the effects of ions and chemical compounds on enzyme stability and activity were assessed.














Similar content being viewed by others
References
Abbasian SS, Ghaznavi Rad E, Akbari N, Zolfaghari MR, Abtahi H (2015) Overexpression and enzymatic assessment of antigenic fragments of hyaluronidase recombinant protein from streptococcus pyogenes. Jundishapur J Microbiol 8(1):e13653
Abeldenov S, Kirillov S, Nurmagambetova A, Kiribayeva A, Silayev D, Khassenov B (2014) Expression, purification and biochemical characterization of recombinant phosphohydrolase appa in Escherichia coli. Eurasian J Appl Biotechnol. https://doi.org/10.11134/btp.3.2014.9
Akbarzadeh A, Dehnavi E, Aghaeepoor M, Amani J (2015) Optimization of recombinant expression of synthetic bacterial phytase in Pichia pastoris using response surface methodology. Jundishapur J Microbiol 8(12):e27553
Awad GE, Helal MM, Danial EN, Esawy MA (2014) Optimization of phytase production by Penicillium purpurogenum GE1 under solid state fermentation by using Box–Behnken design. Saudi J Biol Sci 21(1):81–88
Bawane R, Tantwai K, Rajput L, Kadam-Bedekar M, Kumar S, Gontia I, Tiwari S (2011) Molecular analysis of phytase gene cloned from Bacillus subtilis. Adv Stud Biol 3(3):103–110
Berry DF, Shang C, Zelazny LW (2009) Measurement of phytase activity in soil using a chromophoric tethered phytic acid probe. Soil Biol Biochem 41(2):192–200. https://doi.org/10.1016/j.soilbio.2008.09.011
Clark EDB (1998) Refolding of recombinant proteins. Curr Opin Biotechnol 9(2):157–163
Clark EDB, Schwarz E, Rudolph R (1999) [15] Inhibition of aggregation side reactions during in vitro protein folding. Methods Enzymol 309:217–236
Dai F, Qiu L, Ye L, Wu D, Zhou M, Zhang G (2011) Identification of a phytase gene in barley (Hordeum vulgare L.). PLoS ONE 6(4):e18829
Dharmsthiti S, Chalermpornpaisarn S, Kiatiyajarn M, Chanpokapaiboon A, Klongsithidej Y, Techawiparut J (2005) Phytase production from Pseudomonas putida harbouring Escherichia coli appA. Process Biochem 40(2):789–793
El-Toukhy NM, Youssef AS, Mikhail MG (2013) Isolation, purification and characterization of phytase from Bacillus subtilis MJA. Afr J Biotechnol 12(20):2957–2967
Escobin-Mopera L, Ohtani M, Sekiguchi S, Sone T, Abe A, Tanaka M, Meevootisom V, Asano K (2012) Purification and characterization of phytase from Klebsiella pneumoniae 9-3B. J Biosci Bioeng 113(5):562–567
Farjadi V, Abtahi H, Zolfaghari MR, Soufian S, Hasanzadeh L (2013) Expression, purification and evaluation of antigenicity of CagA antigenic fragment of Helicobacter pylori. Jundishapur J Microbiol. 6(9):1–6
Fei B, Cao Y, Xu H, Li X, Song T, Fei Z,.. . Cao Y (2013) AppA C-terminal plays an important role in its thermostability in Escherichia coli. Curr Microbiol 66(4):374–378
Gleiter S, Bardwell JCA (2008) Disulfide bond isomerization in prokaryotes. Biochim Biophys Acta (BBA)-Mol Cell Res 1783(4):530–534. https://doi.org/10.1016/j.bbamcr.2008.02.009
Golovan S, Wang G, Zhang J, Forsberg CW (1999) Characterization and overproduction of the Escherichia coli appA encoded bifunctional enzyme that exhibits both phytase and acid phosphatase activities. Can J Microbiol 46(1):59–71
Greiner R, Farouk A-E (2007) Purification and characterization of a bacterial phytase whose properties make it exceptionally useful as a feed supplement. Protein J 26(7):467–474
Hasanzadeh L, Ghaznavi-Rad E, Soufian S, Farjadi V, Abtahi H (2013) Expression and antigenic evaluation of vacA antigenic fragment of Helicobacter pylori. Iran J Basic Med Sci 16(7):835–840
Idicula-Thomas S, Balaji PV (2007) Protein aggregation: a perspective from amyloid and inclusion-body formation. Curr Sci 92(6):1–6
Jorquera M, Martínez O, Maruyama F, Marschner P, de la L Mora M (2008) Current and future biotechnological applications of bacterial phytases and phytase-producing bacteria. Microbes Environ 23(3):182–191
Kerovuo JS (2000) A novel phytase from Bacillus: characterization and production of the enzyme. Helsingin Yliopisto (Finland), Ann Arbor
Kerovuo J, Lauraeus M, Nurminen P, Kalkkinen N, Apajalahti J (1998) Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl Environ Microbiol 64(6):2079–2085
Lee S, Kim T, Stahl CH, Lei XG (2005) Expression of Escherichia coli AppA2 phytase in four yeast systems. Biotechnol Lett 27(5):327–334
Lei XG, Porres JM (2003) Phytase enzymology, applications, and biotechnology. Biotechnol Lett 25(21):1787–1794
Mirjamali NA-S, Soufian S, Molaee N, Sadoogh Abbasian S, Abtahi H (2014) Cloning and expression of the enzymatic region of Streptococcal hyaluronidase. Iran J Basic Med Sci 17(9):667–672
Mullaney E, Sethumadhavan K, Boone S, Lei XG, Ullah AH (2012) Elimination of a disulfide bridge in Aspergillus niger NRRL 3135 Phytase (PhyA) enhances heat tolerance and optimizes its temperature versus activity profile. Adv Biol Chem 2:372–378
Phan J, Yamout N, Schmidberger J, Bottomley SP, Buckle AM (2011) Refolding your protein with a little help from REFOLD. In: Hill A, Barnham K, Bottomley S, Cappai R (eds) Protein folding, misfolding, and disease: methods and protocols. Humana Press, Totowa, pp 45–57
Rao D, Rao K, Reddy V (2008) Cloning and expression of Bacillus phytase gene (phy) in Escherichia coli and recovery of active enzyme from the inclusion bodies. J Appl Microbiol 105(4):1128–1137
Rocky-Salimi K, Hashemi M, Safari M, Mousivand M (2016) A novel phytase characterized by thermostability and high pH tolerance from rice phyllosphere isolated Bacillus subtilis BS 46. J Adv Res 7(3):381–390
Rodriguez E, Han Y, Lei XG (1999a) Cloning, sequencing, and expression of anEscherichia coliacid phosphatase/phytase gene (appA2) isolated from pig colon. Biochem Biophys Res Commun 257(1):117–123
Rodriguez E, Porres JM, Han Y, Lei XG (1999b) Different sensitivity of recombinant Aspergillus niger phytase (r-PhyA) and Escherichia coli pH 2.5 acid phosphatase (r-AppA) to trypsin and pepsinin vitro. Arch Biochem Biophys 365(2):262–267
Rodriguez E, Wood ZA, Karplus PA, Lei XG (2000) Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/phytase expressed in Pichia pastoris. Arch Biochem Biophys 382(1):105–112
Sandberg A-S, Andersson H, Carlsson N-G, Sandström B (1987) Degradation products of bran phytate formed during digestion in the human small intestine: effect of extrusion cooking on digestibility. J Nutr 117:2061–2065
Shao N, Huang H, Meng K, Luo H, Wang Y, Yang P, Yao B (2008) Cloning, expression, and characterization of a new phytase from the phytopathogenic bacterium Pectobacterium wasabiae DSMZ 18074. [Research Support, Non-U S Gov’t]. J Microbiol Biotechnol 18(7):1221–1226
Sharma R, Kumar P, Kaushal V, Das R, Kumar Navani N (2018) A novel protein tyrosine phosphatase like phytase from Lactobacillus fermentum NKN51: cloning, characterization and application in mineral release for food technology applications. Bioresour Technol 249:1000–1008
Tan H, Tang J, Li X, Liu T, Miao R, Huang Z, Wang Y, Gan B, Peng W (2017) Biochemical characterization of a psychrophilic phytase from an artificially cultivable morel Morchella importuna. J Microbiol Biotechnol 27(12):2180–2189
Ullah AH, Sethumadhavan K, Mullaney EJ (2005) Monitoring of unfolding and refolding in fungal phytase (phyA) by dynamic light scattering. Biochem Biophys Res Commun 327(4):993–998
Vasudevan UM, Salim SHB, Pandey A (2011) A comparative analysis of recombinant expression and solubility screening of two phytases in E. coli. Food Technol Biotechnol 49(3):304
Wu T-H, Chen C-C, Cheng Y-S, Ko T-P, Lin C-Y, Lai H-L, Huang TY, Liu JR, Guo R-T (2014) Improving specific activity and thermostability of Escherichia coli phytase by structure-based rational design. J Biotechnol 175:1–6
Xiong AS, Yao QH, Peng RH, Han PL, Cheng ZM, Li Y (2005) High level expression of a recombinant acid phytase gene in Pichia pastoris. J Appl Microbiol 98(2):418–428
Yao MZ, Zhang YH, Lu WL, Hu MQ, Wang W, Liang AH (2012) Phytases: crystal structures, protein engineering and potential biotechnological applications. J Appl Microbiol 112(1):1–14
Zinin NV, Serkina AV, Gelfand MS, Shevelev AB, Sineoky SP (2004) Gene cloning, expression and characterization of novel phytase from Obesumbacterium proteus. FEMS Microbiol Lett 236(2):283–290
Acknowledgements
This study was conducted with financial assistance from Arak University of medical sciences, Iran, and we are grateful for their invaluable contribution to this study. This study was the thesis (no: 2351) by Ms. Malihe Hallaji, the master student of Medical Biotechnology at Arak university of medical sciences, Iran. Its ethical code from the ethics committee of Arak University of medical sciences, Arak, Iran is IR.ARAKMU.REC.1394.195.
Funding
This study was conducted with financial assistance from Molecular and Medicine Research Center, Arak University of Medical Sciences, Arak, Iran and we are grateful for their invaluable contribution to this study.
Author information
Authors and Affiliations
Contributions
All authors had equal contributions to the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hallaji, M., Parhamfar, M., Raoufi, E. et al. Cloning and High-Level Expression of the Enzymatic Region of Phytase in E. coli. Int J Pept Res Ther 25, 1431–1439 (2019). https://doi.org/10.1007/s10989-018-9788-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-018-9788-4