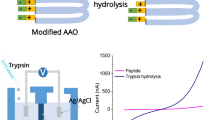
Abstract
Trypsin is an enzyme that is included in the hydrolysis class of enzymes. Trypsin catalyzes the cleavage of proteins from a serine residue and is found in the digestive system of vertebrates. The cystic fibrosis, which is an autosomal recessive disorder, is associated with the trypsin deficiency. The meconium ileus is caused by this deficiency. Beside the clinical significance of trypsin, it is also used in many industrial applications, such as tissue culture labs, milk industry, proteomics, and different kinds of food production processes. In this study, a glucose oxidase based electrochemical biosensing system was constructed for sensitive and accurate determination of trypsin activity in different samples. A novel working principle was operated in the reported biosensor, which is based on the trypsin degradation ratio in the bioactive layer of the biosensor. Detailed optimization and characterization experiments were carried out to obtain the best electrochemical signals. The system introduced a linear determination range for trypsin activity between 6.65 and 33.25 U/ml. The biosensor was also used in the analysis of the trypsin activities of real samples.





Similar content being viewed by others
References
Anderson E, Sze KWC, Sathe SK (1995) New colorimetric method for the detection and quantitation of proteolytic enzyme activity. J Agric Food Chem 43:1530–1534
Aruldoss V, Kalaichelvan PT (2014) Biosensor application of extracellular catalase from Ganoderma lucidum AVK-1 and Acinetobacter calcoaceticus AV-6 and comparative study of commercial catalase from Bovine liver catalase. J Pharm Biol Sci 9:51–54
Asgeirsson B, Cekan P (2006) Microscopic rate-constants for substrate binding and acylation in cold-adaptation of trypsin I from Atlantic cod. FEBS Lett 580:4639–4644
Audrézet MP, Munck A, Scotet V, Claustres M, Roussey M, Delmas D, Férec C, Desgeorges M (2015) Comprehensive CFTR gene analysis of the French cystic fibrosis screened newborn cohort: implications for diagnosis, genetic counseling, and mutation-specific therapy. Genet Med 17:108–116
Bai Y, Xu T, Luong JHT, Cui H (2014) Direct electron transfer of glucose oxidase-boron doped diamond ınterface: a new solution for a classical problem. Anal Chem 86:4910–4918
Barsan MM, David M, Florescu M, Ţugulea L, Brett CMA (2014) A new self-assembled layer-by-layer glucose biosensor based on chitosan biopolymer entrapped enzyme with nitrogen doped graphene. Bioelectrochemistry 99:46–52
Besbes E, Bail AL, Seetharaman K (2014) Effect of baking conditions on properties of starch ısolated from bread crumbs: pasting properties, ıodine complexing ability, and X-ray patterns. Food Bioprocess Technol 7:3407–3415
Bond JS, Beynon RJ (1989) Commercially available proteases, proteolytic enzymes, a practical approach. Oxford A3:317–330
Calvano CD, Ceglie CD, Zambonin CG (2014) proteomic analysis of complex protein samples by MALDI–TOF Mass spectrometry. Methods Mol Biol 1129:365–380
Canbaz MÇ, Sezgintürk MK (2014) Fabrication of a highly sensitive disposable immunosensor based on indium tin oxide substrates for cancer biomarker detection. Anal Biochem 446:9–18
Canbaz MÇ, Simsek ÇS, Sezgintürk MK (2014) Electrochemical biosensor based on self-assembled monolayers modified with gold nanoparticles for detection of HER-3. Anal Chim Acta 814:31–38
Cheisona SC, Leeba E, Toro-Sierrac J, Kulozikc U (2011) Influence of hydrolysis temperature and pH on the selective hydrolysis of whey proteins by trypsin and potential recovery of native alpha-lactalbumin. Int Dairy J 21:166–171
Collins SA, Walker WT, Lucas JS (2014) Genetic testing in the diagnosis of primary ciliary dyskinesia: state-of-the-art and future perspectives. J Clin Med 3:491–503
Corey DR, Willett WS, Coombs GS, Craik CS (1995) Trypsin specificity increased through substrate-assisted catalysis. Biochemistry 34:11521–11527
Cruz-Huerta E, García-Nebot MJ, Miralles B, Recio I, Amigo L (2014) Caseinophosphopeptides released after tryptic hydrolysis versus simulated gastrointestinal digestion of a casein-derived by-product. Food Chem 168:648–655
De Castro RJS, Bagagli MP, Sato HH (2015) Improving the functional properties of milk proteins: focus on the specificities of proteolytic enzymes. Curr Opin Food Sci 1:64–69
De Ceglie C, Calvano CD, Zambonin CG (2014) determination of hidden hazelnut oil proteins in extra virgin olive oil by cold acetone precipitation followed by ın-solution tryptic digestion and MALDI-TOF-MS analysis. J Agric Food Chem 62:9401–9409
Diecke S, Lisowski L, Kooreman NG, Wu JC (2014) Second generation codon optimized minicircle (CoMiC) for nonviral reprogramming of human adult fibroblasts. Methods Mol Biol 1181:1–13
Ding X, Ge D, Yang K (2014) Colorimetric protease assay by using gold nanoparticles and oligopeptides. Sens Actuators, B 201:234–239
Dittrich J, Becker S, Hecht M, Ceglarek U (2015) Sample preparation strategies for targeted proteomics via proteotypic peptides in human blood using liquid chromatography tandem mass spectrometry. Proteom Clin Appl 9:5–16
Fadnavis NW, Babu RL, Deshpande A (1998) Reactivity of trypsin in reverse micelles: pH-effects on the W0 versus enzyme activity profiles. Biochimie 80:1025–1030
Flannery AV, Beynon RJ, Bond JS (1989) Proteolysis of proteins for sequencing analysis and peptide mapping, proteolytic enzymes. A Pract Approach, Oxford, p 45
Goktuğ T, Sezgintürk MK, Dinçkaya E (2005) Glucose oxidase-beta-galactosidase hybrid biosensor based on glassy carbon electrode modified with mercury for lactose determination. Anal Chim Acta 551:51–56
Graham SS, Jelliss PA (2014) Brominated ferracarboranes as diffuse redox mediators for glucose oxidase bioanodes. Inorg Chim Acta 410:195–201
Halim A, Carlsson MC, Madsen CB, Brand S, Møller SR, Olsen CE, Vakhrushev SY, Brimnes J, Wurtzen PA, Ipsen H, Petersen BL, Wandall HH (2015) Glycoproteomic analysis of seven major allergenic proteins reveals novel post-translational modifications. Mol Cell Proteom 14:191–204
Hong ML, Li LJ, Han HX, Chu X (2014) A label-free fluorescence assay for trypsin based on the electron transfer between oligonucleotide-stabilized Ag nanoclusters and cytochrome c. Anal Sci 30:811–815
Hu L, Han S, Parveen S, Yuan Y, Zhang L, Xu G (2012) Highly sensitive fluorescent detection of trypsin based on BSA-stabilized gold nanoclusters. Biosens Bioelectron 32:297–299
Irshad I, Kanekanian A, Peters A, Masud T (2015) Antioxidant activity of bioactive peptides derived from bovine casein hydrolysate fractions. J Food Sci Technol 52:231–239
Izumi Y, Sato Y, Kakui K, Tatsumi K, Fujiwara H, Konishi I (2011) Prenatal treatment of meconium peritonitis with urinary trypsin inhibitor. Ultrasound Obstet Gynecol 37:366–368
Jolley ME (1996) Fluorescence polarization assays for the detection of proteases and their ınhibitors. J Biomol Screen 1:33–38
Kimland M, Russick C, Marks WH, Borgström A (1989) Immunoreactive anionic and cationic trypsin in human serum. Clin Chim Acta 184:31–46
LaRusch J, Lozano-Leon A, Stello K, Moore A, Muddana V, O’Connell M, Diergaarde B, Yadav D, Whitcomb DC (2015) The common chymotrypsinogen C (CTRC) Variant G60G (C.180T) ıncreases risk of chronic pancreatitis but not recurrent acute pancreatitis in a North American population. Clin Transl Gastroenterol 6:1–9
Lee YM, Jeong Y, Kang HJ, Chung SJ, Chung BH (2009) Cascade enzyme-linked immunosorbent assay (CELISA). Biosens Bioelectron 25:332–337
Lili Y, Yang Z, Chenyi H, Hui W, Yayun Y, Chusen H, Nengqin J (2015) Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem 176:22–26
Lin D, Tang T, Harrison DJ, Lee WE, Jemere AB (2015) A regenerating ultrasensitive electrochemical impedance immunosensor for the detection of adenovirus. Biosens Bioelectron 68:129–134
Liu Y, Chen J, Xu M, Zhao G (2014) A novel photoelectrochemical platform for detection of protease. Int J Electrochem Sci 9:4014–4023
Nguyen-Ngoc KV, Shamir E, Huebner RJ, Beck JN, Cheung KJ, Ewald AJ (2015) 3D culture assays of murine mammary branching morphogenesis and epithelial invasion. Methods Mol Biol 1189:135–162
Ou L, Li X, Li L, Liu H, Suna A, Liu K (2015) A sensitive assay for trypsin using poly(thymine)-templated copper nanoparticles as fluorescent probes. Analyst 140:1871–1875
Pan Y, Ye M, Zheng H, Cheng K, Sun Z, Liu F, Liu J, Wang K, Qin H, Zou H (2014) Trypsin-catalyzed n-terminal labeling of peptides with stable ısotope-coded affinity tags for proteome analysis. Anal Chem 86:1170–1177
Rawlings ND, Barrett AJ (1994) Families of serine peptidases. Methods Enzymol 244:19–61
Sezgintürk MK (2011) A new impedimetric biosensor utilizing VEGF receptor-1 (Flt-1): early diagnosis of vascular endothelial growth factor in breast cancer. Biosens Bioelectron 26:4032–4039
Sezgintürk MK, Dinçkaya E (2003) A novel amperometric biosensor based on spinach (Spinacia oleracea) tissue homogenate for urinary oxalate determination. Talanta 59:545–551
Sezgintürk MK, Goktuğ T, Dinçkaya E (2005) Detection of benzoic acid by an amperometric inhibitor biosensor based on mushroom tissue homogenate. Food Technol Biotechnol 43:329–334
Shih PM, Liu TK, Tan KT (2013) Fluorescence amplified detection of proteases by the catalytic activation of a semisynthetic sensor. Chem Commun 49:6212–6214
Silvaa JF, Espósitoa TS, Marcuschia M, Ribeiroa K, Cavallib RO, Oliveirac V, Bezerraa RS (2011) Purification and partial characterisation of a trypsin from the processing waste of the silver mojarra (Diapterus rhombeus). Food Chem 129:777–782
Simonov AN, Grosse W, Mashkina EA, Bethwaite B, Tan J, Abramson D, Wallace GG, Moulton SE, Bond AM (2014) New insights into the analysis of the electrode kinetics of flavin adenine dinucleotide redox center of glucose oxidase ımmobilized on carbon electrodes. Langmuir 30:3264–3273
Soltanizadeh N, Mirmoghtadaie L, Nejati F, Najafabadi LI, Heshmati MK, Jafari M (2014) Solid-State Protein-Carbohydrate Interactions and Their Application in the Food Industry. Compr Rev Food Sci Food Saf 13:860–870
Stoytcheva M, Zlatev R, Cosnier S, Arredondo M, Valdez B (2013) High sensitive trypsin activity evaluation applying a nanostructured QCM-sensor. Biosens Bioelectron 41:862–866
Vestling MM, Murphy CM, Fenselau C (1990) Recognition of trypsin autolysis products by high-performance liquid chromatography and mass spectrometry. Anal Chem 62:2391–2394
Vipperla K, O’Keefe SJ, Forsmark CE, Gardner TB (2015) Nutrition in severe acute pancreatitis. In: Forsmark CE, Gardner TB (eds) Prediction and management of severe acute pancreatitis. Springer, New York, pp 123–132
Wael KD, Belder SD, Pilehar S, Steenberge GV, Herrebout W, Heering HA (2012) Enzyme-gelatin electrochemical biosensors: scaling down. Biosensors 2:101–113
Wang W, Zhao K, Ren W, Liu T, Zhang, Ouyang P, Kaplan S. (2014) Enzymatic extraction and characteristics of collagen fibers from cattle skin. Proceedings of the 2012 international conference on applied biotechnology (ICAB 2012), vol 251,Springer, Berlin, pp 1441–1448
Wang H, Broker TR, Chow LT (2015) Robust HPV-18 production in organotypic cultures of primary human keratinocytes. Methods Mol Biol 1249:93–109
Yu C, Merza M, Luo L, Thorlacius H (2014) Inhibition of Ras signalling reduces neutrophil infiltration and tissue damage in severe acute pancreatitis. Eur J Pharmacol 746:245–251
Yue GG, Kwok H, Lee JK, Jiang L, Chan K, Cheng L, Wong EC, Leung P, Fung K, Lau CB (2015) Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J Funct Foods 15:243–253
Zhang X, Shen L, Wang M, Siqin G, Tong Z, Xu R, Zhang D, Ma J, Liu L (2014a) A novel glucose biosensor constructed by glucose oxidase immobilized with methylene blue intercalated layered lanthanum niobic acid nanocomposite. Mater Lett 135:39–42
Zhang W, Zhang P, Zhang S, Zhu C (2014b) Label-free and real-time monitoring of trypsin activity in living cells by quantum-dot-based fluorescent sensors. Anal Methods 6:2499–2505
Zheng F, Wu J, Zhao G (2012) Peptide–quantum dot bioconjugates for label-free trypsin detection based on the exciton energy transfer. Anal Methods 4:3932–3936
Acknowledgements
This study was financially supported by The Scientific and Technological Research Council of Turkey (TUBİTAK) in the frame of the project numbered 113Z678.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Belfin Işık and Mustafa Kemal Sezgintürk declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Human and Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Işık, B., Sezgintürk, M.K. Quantification of Trypsin Activity by a New Biosensing System Based on the Enzymatic Degradation and the Destructive Nature of Trypsin. Int J Pept Res Ther 23, 313–322 (2017). https://doi.org/10.1007/s10989-016-9563-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-016-9563-3