Abstract
Context
Crop rotations, within-field mixtures, and landscape mosaics including susceptible and resistant crops are three commonly adopted crop diversification strategies that can limit crop epidemics. Typically, the effects of crop diversification at these three scales have been studied separately, on single pathogen species, and with low environmental variability.
Objectives
We aim to compare the disease-limitation effect of these three types of crop diversification on two highly damaging fungal pathogens of wheat Puccinia recondita (WLR) and Zymoseptoria tritici (STB) and under varying weather conditions (warmer or cooler climate for WLR, wetter or drier conditions for STB).
Methods
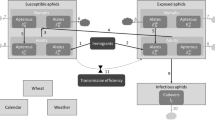
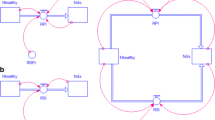
We built a dynamic mathematical model of epidemics at the field scale (based on classical Susceptible-Exposed-Infectious-Removed epidemiological models) embedded in a spatially explicit landscape grid framework. We use it to simulate an agricultural landscape in which diversification translates into different proportions of wheat and resistant crops in the landscape.
Results
In our simulations, for both pathogens and in all weather conditions, within-field crop mixtures had the greatest impact in limiting epidemics, crop rotations were second-best, while landscape mosaics were the least effective. We also found that the threshold above which further addition of resistant plants to crop mixtures would not cause further disease limitation to be dependent on weather conditions. The more favorable the weather is for pathogens the more resistant plants are required.
Conclusions
Our findings imply that interactions between spatial scale of crop diversification, pathogen characteristics and weather conditions should be considered in order to maximize benefits from disease-regulation properties of diversified cropping systems under climate change.




Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. The Matlab implementation of the model is available upon request by e-mail to the corresponding author: pierre-antoine.precigout@inrae.fr.
References
Azzimonti G, Lannou C, Sache I, Goyeau H (2013) Components of quantitative resistance to leaf rust in wheat cultivars: diversity, variability and specificity. Plant Pathol 62:970–981
Baccar R, Fournier C, Dornbusch T et al (2011) Modelling the effect of wheat canopy architecture as affected by sowing density on Septoria tritici epidemics using a coupled epidemicvirtual plant model. Ann Bot 108:1179–1194
Bancal MO, Robert C, Ney B (2007) Modelling wheat growth and yield losses from late epidemics of foliar diseases using loss of green leaf area per layer and pre-anthesis reserves. Ann Bot 100:777–789
Bargués-Ribera M, Gokhale CS (2020) Eco-evolutionary agriculture: host-pathogen dynamics in crop rotations. PLoS Comput Biol 16:e1007546
Barillot R, Combes D, Chevalier V et al (2012) How does pea architecture influence light sharing in virtual wheat-pea mixtures? A simulation study based on pea genotypes with contrasting architectures. AoB Plants 2012:pls08–pls08
Barot S, Allard V, Cantarel A et al (2017) Designing mixtures of varieties for multifunctional agriculture with the help of ecology. Rev Agron Sustain Dev. https://doi.org/10.1007/s13593-017-0418-x
Béasse C, Ney B, Tivoli B (2000) A simple model of pea (Pisum sativum) growth affected by Mycosphaerella pinodes. Plant Pathol 49:187–200
Bedoussac L, Justes E (2010) The efficiency of a durum wheat-winter pea intercrop to improve yield and wheat grain protein concentration depends on N availability during early growth. Plant Soil 330:19–35
Beillouin D, Ben-Ari T, Makowski D (2019) Evidence map of crop diversification strategies at the global scale. Environ Res Lett 14:123001
Beillouin D, Ben-Ari T, Malézieux E et al (2021) Positive but variable effects of crop diversification on biodiversity and ecosystem services. Glob Chang Biol. https://doi.org/10.1111/gcb.15747
Ben M’Barek S, Karisto P, Abdedayem W et al (2020) Improved control of septoria tritici blotch in durum wheat using cultivar mixtures. Plant Pathol. https://doi.org/10.1111/ppa.13247
Benbi DK (1994) Prediction of leaf area indices and yields of wheat. J Agric Sci 122:13–20
Bhattacharya S (2017) Wheat rust back in Europe. Nature 542:145–146
Boland GJ, Melzer MS, Hopkin A et al (2004) Climate change and plant diseases in Ontario. Can J Plant Pathol 26:335–350
Borg J, Kiær LP, Lecarpentier C et al (2018) Unfolding the potential of wheat cultivar mixtures: a meta-analysis perspective and identification of knowledge gaps. F Crop Res 221:298–313
Brown JKM, Hovmøller MS (2002) Aerial dispersal of fungi on the global and continental scales and its consequences for plant disease. Science 297:537–541
Bullock DG (1992) Crop rotation. CRC Crit Rev Plant Sci 11:309–326
Burdon JJ, Barrett LG, Rebetzke G, Thrall PH (2014) Guiding deployment of resistance in cereals using evolutionary principles. Evol Appl 7:609–624
Calonnec A, Goyeau H, De V-P (1996) Effects of induced resistance on infection efficiency and sporulation of Puccinia striiformis on seedlings in varietal mixtures and on field epidemics in pure stands. Eur J Plant Pathol 102:733–741
Caubel J, Launay M, Lannou C, Brisson N (2012) Generic response functions to simulate climate-based processes in models for the development of airborne fungal crop pathogens. Ecol Modell 242:92–104
Cotuna O, Paraschivu M, Paraschivu M, Olaru L (2018) Influence of crop management on the impact of zymoseptoria tritici in winter wheat in the context of climate change : an overview. Res J Agric Sci 50:69–76
Duvivier M, Dedeurwaerder G, Bataille C et al (2016) Real-time PCR quantification and spatio-temporal distribution of airborne inoculum of Puccinia triticina in Belgium. Eur J Plant Pathol 145:405–420
El Jarroudi M, Kouadio L, Delfosse P, Tychon B (2014) Brown rust disease control in winter wheat: I. Exploring an approach for disease progression based on night weather conditions. Environ Sci Pollut Res 21:4797–4808
Evers JB, Van Der Werf W, Stomph TJ et al (2019) Understanding and optimizing species mixtures using functional-structural plant modelling. J Exp Bot 70:2381–2388
Eversmeyer MG, Kramer CL (1998) Models of early spring survival of wheat leaf rust in the central great plains. Plant Dis 82:987–991
Eyal Z (1987) The septoria diseases of wheat: concepts, methods and management
Fabre F, Rousseau E, Mailleret L, Moury B (2015) Epidemiological and evolutionary management of plant resistance: optimizing the deployment of cultivar mixtures in time and space in agricultural landscapes. Evol Appl 8:919–932
Finckh MR, Gacek ES, Czembor HJ, Wolfe MS (1999) Host frequency and density effects on powdery mildew and yield in mixtures of barley cultivars. Plant Pathol 48:807–816
Finckh MR, Gacek ES, Goyeau H et al (2000) Cereal variety and species mixtures in practice, with emphasis on disease resistance. Agronomie 20:813–837
Forman RTT (1995) Some general principles of landscape and regional ecology. Landsc Ecol 10:133–142
Forsman K, Poutala T (1997) Crop management effects on pre- and post-anthesis changes in leaf area index and leaf area duration and their contribution to grain yield and yield components in spring cereals. J Agron Crop Sci 61:47–61
Frezal L, Robert C, Bancal M-O, Lannou C (2009) Local dispersal of Puccinia triticina and wheat canopy structure. Phytopathology 99:1216–1224
Garin G, Fournier C, Andrieu B et al (2014) A modelling framework to simulate foliar fungal epidemics using functional-structural plant models. Ann Bot 114:795–812
Garin G, Pradal C, Fournier C et al (2018) Modelling interaction dynamics between two foliar pathogens in wheat: a multi-scale approach. Ann Bot 121:927–940
Gaudio N, Louarn G, Barillot R et al (2022) Exploring complementarities between modelling approaches that enable upscaling from plant community functioning to ecosystem services as a way to support agroecological transition. In Silico Plants 4:1–13
Gilligan CA (2008) Sustainable agriculture and plant diseases: an epidemiological perspective. Philos Trans R Soc B Biol Sci 363:741–759
Gouache D, Bensadoun A, Brun F et al (2013) Modelling climate change impact on septoria tritici blotch (STB) in France: accounting for climate model and disease model uncertainty. Agric Meteorol 170:242–252
Grabow BS, Shah DA, Dewolf ED (2016) Environmental conditions associated with stripe rust in kansas winter wheat. Plant Dis 100:2306–2312
Hinzman LD, Bauer ME, Daughtry CST (1986) Effects of nitrogen-fertilization on growth and reflectance characteristics of winter-wheat. Remote Sens Environ 19:47–61
Hossard L, Souchere V, Jeuffroy MH (2018) Effectiveness of field isolation distance, tillage practice, cultivar type and crop rotations in controlling phoma stem canker on oilseed rape. Agric Ecosyst Environ 252:30–41
Huang J, Sedano F, Huang Y et al (2016) Assimilating a synthetic Kalman filter leaf area index series into the WOFOST model to improve regional winter wheat yield estimation. Agric for Meteorol 216:188–202
Junk J, Kouadio L, Delfosse P, El Jarroudi M (2016) Effects of regional climate change on brown rust disease in winter wheat. Clim Change 135:439–451
Juskiw PE, Helm JH, Salmon DF (2000) Competitive ability in mixtures of small grain cereals. Crop Sci 40:159–164
Knops JMH, Tilman D, Haddad NM et al (1999) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett 2:286–293
Kolster P, Munk L, Stolen O (1989) Disease severity and grain yield in barley multilines with resistance to powdery mildew. Crop Sci 29:1459–1463
Kovats RS, Valentini R, Bouwer LM et al (2015) IPCC Report 2014 - Europe. In: Climate change 2014: impacts, adaptation and vulnerability. Part B: Regional aspects. Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change, pp 1267–1326
Le Gal A, Robert C, Accatino F et al (2020) Modelling the interactions between landscape structure and spatio-temporal dynamics of pest natural enemies: implications for conservation biological control. Ecol Modell 420:108912
Lescourret F, Magda D, Richard G et al (2015) A social-ecological approach to managing multiple agro-ecosystem services. Curr Opin Environ Sustain 14:68–75
Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate trends and global crop production since 1980. Science 333:616–620
Loughman R, Wilson RE, Thomas GJ (1996) Components of resistance to Mycosphaerella graminicola and Phaeosphaeria nodorum in spring wheats. Euphytica 89:377–385
Luck J, Spackman M, Freeman A et al (2011) Climate change and diseases of food crops. Plant Pathol 60:113–121
Madden LV, Hughes G, van den Bosch F (2007) The study of plant disease epidemics. The American Phytopathological Society, St. Paul, MN
Malagoli P, Naudin C, Vrignon-Brenas S et al (2020) Modelling nitrogen and light sharing in pea-wheat intercrops to design decision rules for N fertilisation according to farmers’ expectations. F Crop Res. https://doi.org/10.1016/j.fcr.2020.107865
MATLAB (2020) 9.7.0.1190202 (R2020a), Natick, Massachusetts. The MathWorks Inc
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol 40:349–379
Meynard JM, Charrier F, Fares M et al (2018) Socio-technical lock-in hinders crop diversification in France. Agron Sustain Dev. https://doi.org/10.1007/s13593-018-0535-1
Mommer L, Cotton TEA, Raaijmakers JM et al (2018) Lost in diversity: the interactions between soil-borne fungi, biodiversity and plant productivity. New Phytol 218:542–553
Morais D, Sache I, Suffert F, Laval V (2016) Is the onset of septoria tritici blotch epidemics related to the local pool of ascospores? Plant Pathol 65:250–260
Mundt CC (2002) Use of multiline cultivars and cultivar mixtures for disease management. Annu Rev Phytopathol 40:381–410
Mundt CC, Brophy LS (1988) Influence of number of host genotype units on the effectiveness of host mixture for disease control: a modelling approach. Phytopathology 1:1087–1094
Mundt C, Leonard K (1986) Effect of host genotype unit area on development of focal epidemics of bean rust and common maize rust in mixtures of resistant and susceptible plants. Phytopathology 76:895–900
Mundt CC, Sackett KE, Wallace LD (2011) Landscape heterogeneity and disease spread: experimental approaches with a plant pathogen. Ecol Appl 21:321–328
Newton AC (2009) Plant disease control through the use of variety mixtures. In: Disease control in crops: biological and environmentally friendly approaches. Wiley-Blackwell, pp 162–171
Nicholls C, Altieri M (2004) Designing species-rich, pest-suppressive agroecosystems through habitat management. Agronomy 43:49–62
O’Connell MG, O’Leary GJ, Whitfield DM, Connor DJ (2004) Interception of photosynthetically active radiation and radiation-use efficiency of wheat, field pea and mustard in a semi-arid environment. F Crop Res 85:111–124. https://doi.org/10.1016/S0378-4290(03)00156-4
Papaïx J, Adamczyk-Chauvat K, Bouvier A et al (2014a) Pathogen population dynamics in agricultural landscapes: The Ddal modelling framework. Infect Genet Evol 27:1–12
Papaïx J, Rimbaud L, Burdon JJ et al (2018) Differential impact of landscape-scale strategies for crop cultivar deployment on disease dynamics, resistance durability and long-term evolutionary control. Evol Appl. https://doi.org/10.1111/eva.12570
Papaïx J, Touzeau S, Monod H, Lannou C (2014b) Can epidemic control be achieved by altering landscape connectivity in agricultural systems? Ecol Modell 284:35–47
Pariaud B, Ravigné V, Halkett F et al (2009) Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathol 58:409–424
Perfect SE, Green JR (2001) Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol 2:101–108
Précigout PA, Claessen D, Makowski D, Robert C (2020a) Does the latent period of leaf fungal pathogens reflect their trophic type? A meta-analysis of biotrophs, hemibiotrophs, and necrotrophs. Phytopathology 110:345–361
Précigout P, Claessen D, Robert C (2017) Crop fertilization impacts epidemics and optimal latent period of biotrophic fungal pathogens. Phytopathology. https://doi.org/10.1094/PHYTO-01-17-0019-R
Précigout PA, Robert C, Claessen D (2020b) Adaptation of biotrophic leaf pathogens to fertilization-mediated changes in plant traits: a comparison of the optimization principle to invasion fitness. Phytopathology 110:1039–1048
Rimbaud L, Papaïx J, Barrett LG et al (2018a) Mosaics, mixtures, rotations or pyramiding: what is the optimal strategy to deploy major gene resistance? Evol Appl 11:1791–1810
Rimbaud L, Papaïx J, Rey JF, Barrett LG, Thrall PH (2018b) Assessing the durability and efficiency of landscape-based strategies to deploy plant resistance to pathogens. PLoS Comput Biol 14
Robert C, Bancal M-O, Ney B, Lannou C (2005) Wheat leaf photosynthesis loss due to leaf rust, with respect to lesion development and leaf nitrogen status. New Phytol 165:227–241
Robert C, Bancal M-O, Nicolas P et al (2004) Analysis and modelling of effects of leaf rust and Septoria tritici blotch on wheat growth. J Exp Bot 55:1079–1094
Robert C, Fournier C, Andrieu B, Ney B (2008) Coupling a 3D virtual wheat (Triticum aestivum) plant model with a Septoria tritici epidemic model (Septo3D): a new approach to investigate plant–pathogen interactions linked to canopy architecture. Funct Plant Biol 35:997–1013
Robert C, Garin G, Abichou M et al (2018) Plant architecture and foliar senescence impact the race between wheat growth and zymoseptoria tritici epidemics. Ann Bot 121:975–989
Roelfs AP, Bushnell WR (1984) The cereal rusts. Origins, specificity, structure, and physiology. Academic Press. vol 1. https://doi.org/10.1016/B978-0-12-148401-9.X5001-8
Sache I (2000) Short-distance dispersal of wheat rust spores by wind and rain. Agronomie 20:757–767
Saint-Jean S, Chelle M, Huber L (2004) Modelling water transfer by rain-splash in a 3D canopy using monte carlo integration. Agric for Meteorol 121:183–196
Saunders DGO, Pretorius ZA, Hovmøller MS (2019) Tackling the re-emergence of wheat stem rust in Western Europe. Commun Biol 2:9–11
Skelsey P, Rossing WAH, Kessel GJT, van der Werf W (2010) Invasion of Phytophthora infestans at the landscape level: how do spatial scale and weather modulate the consequences of spatial heterogeneity in host resistance? Phytopathology 100:1146–1161
Suffert F, Delestre G, Gélisse S (2019) Sexual reproduction in the fungal foliar pathogen Zymoseptoria tritici is driven by antagonistic density dependence mechanisms. Microb Ecol 77:110–123.
Suffert F, Sache I (2011) Relative importance of different types of inoculum to the establishment of Mycosphaerella graminicola in wheat crops in North-West Europe. Plant Pathol 60:878–889
Suffert F, Sache I, Lannou C (2013) Assessment of quantitative traits of aggressiveness in Mycosphaerella graminicola on adult wheat plants. Plant Pathol 62:1330–1341
van Maanen A, Xu XM (2003) Modelling plant disease epidemics. Eur J Plant Pathol 109:669–682
Vidal T, Saint-Jean S, Lusley P et al (2020) Cultivar mixture effects on disease and yield remain despite diversity in wheat height and earliness. Plant Pathol 69:1148–1160
Walklate PJ (1989) Vertical dispersal of plant pathogens by splashing. Part I: the theoretical relationship between rainfall and upward rain splash. Plant Pathol 38:56–63
Wang F, Casulli F (1995) Component analysis of partial resistance to Puccinia recondita f. sp. tritici in some Chinese bread wheat cultivars. Phytopathol Mediterr 34:23–28
West JS, Townsend JA, Stevens M, Fitt BDL (2012) Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. Eur J Plant Pathol 133:315–331
Wolfe W (1985) The current status and prospects of multiline cultivars and variety mixtures for disease resistance. Annu Rev Phytopathol 23:251–273
Xu X, Ma L, Hu X (2019) Overwintering of wheat stripe rust under field conditions in the northwestern regions of China. Plant Dis 103:638–644
Acknowledgements
This work was funded by the French National Research Agency under the Programme “Investissements d’Avenir” under the reference ANR 17 MPGA 0004 and by the National Research Institute for Agriculture, Food and the Environment (INRAE). We thank Doyle McKey and Olivier Dangles for helpful comments during the preparation of the manuscript and English proofreading.
Funding
This work was funded by the French National Research Agency under the Programme “Investissements d’Avenir” under the reference ANR 17 MPGA 0004 and by the National Research Institute for Agriculture, Food and the Environment (INRAE).
Author information
Authors and Affiliations
Contributions
Conceptualization: DC & CR. Model Development: DC, JS & CR. Simulation schedule: DC, JS, P-AP & CR. Simulation Analysis: P-AP, DR, DC & CR. Writing, Review and Editing: P-A.P., D.R. & C.R.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10980_2022_1545_MOESM1_ESM.tif
Supplementary file1 (TIF 1865 kb) Supplementary Fig. 1 Seasonal dynamics of the green Leaf Area Index (LAI) of wheat (solid black line, the susceptible and resistant cultivars share the same LAI) and pea (dotted green line). Time is expressed in degree-days (dd)
10980_2022_1545_MOESM2_ESM.tif
Supplementary file2 (TIF 4464 kb) Supplementary Fig. 2 Coherence tests of the effect of rainfall patterns on STB epidemics. A: seasonal dynamics of crop growth (gLAI: green Leaf Area Index when no epidemics occur; S: surface of susceptible crop remaining healthy) and epidemiological dynamics (E: latent crop leaf surface; I: infectious crop leaf surface) for six successive cropping seasons. B: Total of crop leaf surface infected by STB. Below the figure, we represented the rain comb patterns we used to simulate rainfall in the model, corresponding to transformed data from the Grignon research station recorded between (here) 1994 and 2000. Year 1994–95 was a favourable year for STB, while year 1996–97 was unfavourable. This sequence os part of the 1993–2006 sequence used as average weather condition
10980_2022_1545_MOESM3_ESM.tif
Supplementary file3 (TIF 2764 kb) Supplementary Fig. 3 Coherence tests regarding the starting date of WLR epidemics. Seasonal dynamics of crop growth (gLAI: green Leaf Area Index when no epidemics occur; S: surface of susceptible crop remaining healthy) and epidemiological dynamics (E: latent crop leaf surface; I: infectious crop leaf surface) for A: epidemics starting early (favorable weather conditions for the pathogen); B: average starting date of epidemics (average weather conditions for the pathogen) and C: late starting date of epidemics (unfavorable weather conditions for the pathogen)
10980_2022_1545_MOESM4_ESM.tif
Supplementary file4 (TIF 4654 kb) Supplementary Fig. 4 Combined effect of diversification strategies and within-season spore mortality rate on the intensity of epidemics (AUDPC) of WLR and STB. Here the resistant crop is a partially resistant wheat cultivar. Reference overwintering corresponds to \(\rho\) = 0.01 for WLR and \(\rho\) = 0.002 for STB. High within-season spore mortality rate corresponds to \(\rho\) = 0.05 for WLR and \(\rho\) = 0.008 for STB. Low within-season spore mortality rate corresponds to \(\rho\) = 0.005 for WLR and \(\rho\) = 0.0005 for STB
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Précigout, PA., Renard, D., Sanner, J. et al. Crop mixtures outperform rotations and landscape mosaics in regulation of two fungal wheat pathogens: a simulation study. Landsc Ecol 38, 77–97 (2023). https://doi.org/10.1007/s10980-022-01545-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01545-2