Abstract
Aqueous liquid mixtures play a critical role in many biological and chemical processes. Solutes including sugars, sugar alcohols, carboxylic acids, alcohols and acetone can affect the hydrogen-bonded structure of water and this can be measured using pressure perturbation calorimetry (PPC). In binary water–solute mixtures, Δ(∂CP/∂P)T is a measure of the structure of the water component. At low alcohol concentrations, negative Δ(∂CP/∂P)T values are consistent with clathrate-like water cages around the alkyl moieties. Conversely, when solutes hydrogen bond with water it interferes in the formation of “ice-like” water and is observable as a positive Δ(∂CP/∂P)T. The Δ(∂CP/∂P)T at increasing concentrations of ethanol, acetone and acetic acid in water displayed very different behaviors. Ethanol–water mixtures had three distinct concentration dependent phases; the first, with ethanol surrounded by water molecules, followed by the ethyl groups self-associating breaking the clathrate-like cages, and the ethanol–water network displacing all of the bulk water. Acetic acid–water mixtures display nonlinearity in Δ(∂CP/∂P)T versus acetic acid concentration consistent with acetic acid self-interaction which interferes with acetic acid capacity to disrupt water structure. Acetone-water mixtures display linearity in Δ(∂CP/∂P)T versus acetone concentration which is consistent with acetone’s inability to hydrogen bond with other acetone molecules. The lack of negative Δ(∂CP/∂P)T values in acetic acid-water and acetone-water mixtures suggests there is sufficient self-association between these solutes to prevent clathrate-like water cage formation. PPC can provide invaluable insight into the behavior of aqueous binary mixtures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the techniques that emerged after the advent of ultrasensitive calorimetry in the late 1990s, pressure perturbation calorimetry (PPC) is arguably the least used [1,2,3]. Despite this, previous work by Gary Pielak and co-workers in 2004 changed our understanding of aqueous systems and identified PCC as a resourceful orthogonal technique [4]. They used PPC to refute the hypothesis that ionic and non-polar solutes regulated protein stability in solution by altering the water structure, and that solutes could be described as having “structure-making” (kosmotropic) or “structure-breaking” (chaotropic) properties.
The idea that water could be described as a mixture of lower density highly structured “ice-like” water and higher density less structured water has a long history [5, 6]. The conventional view is that the structured “ice-like” component of water has a tetrahedral hydrogen-bonded configuration, that the less structured component is weakly hydrogen-bonded disordered water, and that the hydrogen bonds in water are constantly forming and breaking [7,8,9,10,11]. The apparent dual nature of water can be observed using PPC. When a pressure is applied to a sample it favors a shift to the denser state and the change in heat associated with the change in hydrogen bond population is measured. The solute induced change to the structure of water was credited with being able to affect the viscosity of water [2, 12] and modulate protein stability [13, 14]. Pielak’s group used the theoretical framework established by Loren Hepler that the thermodynamic equation (∂CP/∂P)T = − T(∂2V/∂T2)P related thermal expansion to the structure of water [15]. This enabled a solutes capacity to make or break water structure to be defined as ∂2V/∂T2 [4]. As such, a drop in (∂CP/∂P)T would be expected when pressure or temperature are raised as both reduce proportion of the lower density ice-like species. PPC analysis by Pielak’s group of diverse range of organic and inorganic solutes in water showed no obvious correlation between (∂CP/∂P)T and protein stability, suggesting that while the presence of solutes did affect the structure of water, this did not correspond with the solute’s ability to alter protein stability [4].
Historically, most PPC research was used to study macromolecules. The majority of the studies were on proteins [16,17,18,19,20,21] with nucleic acids [22, 23], lipids and lipid structures [24,25,26] and synthetic polymers [27, 28] also studied. PPC’s strength was its capacity to study the volumetric properties of macromolecules and colloids. The results are usually expressed in terms of the system’s thermal expansion coefficient (α) or molar expansivity (E). Proteins are inherently complex having a structure that can unfold at higher temperatures, and a solvent accessible surface that is combination of charged, polar and hydrophobic amino acid sidechains. Combined, this makes it a challenge to separate solvation from other volumetric effects [16].
This works follows on from that published by Gary Pielak and his co-workers [4], and aims to determine the strengths and limitation of the PPC technique when studying the interactions between small organic molecules and water, and provide a better understanding of these simple mixtures.
Materials and methods
PPC measurements were obtained using a capillary Nano-DSC (TA Instruments, New Castle, DE, USA). Samples were degassed under vacuum for 20 min at 27 °C to prevent bubble formation during the scan. Heat effects were measured in 0.3 mL samples during alternating pressure pulses from 1 to 4 bar, then from 4 to 1 bar, each pressure change occurred at 1 °C intervals. A heating rate of 0.1 °C min−1 was used from 14 to 36 °C, giving a usable data range of 15–35 °C. NanoAnalyze software (TA Instruments, New Castle, DE, USA) was used to calculate the difference in heat supplied to the sample and the reference cell (ΔQexp). The strategy for operating pressure perturbation calorimetry using a rate of 0.1 °C min−1 has been validated to match those generated under isobaric conditions [22].
The heat associated with the water interacting with the solute (ΔQws) on applying the pressure change is calculated using Eq. 1.
where ΔQexp is the experimentally determined heat, and ΔQdw is the heat released or absorbed by the water when displaced by the solute. ΔQdw is calculated using Eq. 2. In this study, the density of the solute in pure form was used to calculate the volume of the solute (\({V}_{\text{s}}\)). This study is designed to study the changes to the water population without the adjustment for change to the water volume.
\({m}_{\text{s}}\) is the mass fraction of the solute; \({V}_{\text{s}}\) is the volume of the solute and \({\alpha }_{0}\) is the thermal expansion coefficient of water, which is 2.52 × 10–4 K−1 at 298 K [29].
The concentration of the solute used in the initial experiments was 2.0 mass/% to minimize the deviation of the Δ(∂CP/∂P)T values due to solute–solute interactions. Isopropanol is known to form mesoscopic networks above 0.02 mol fraction (6.7 mass/%) so a lower concentration was used to minimize this phenomenon [30].
Results
Low concentrations of organic solutes
The pressure pulse from 1 to 4 bar to the reference and sample cell is accompanied by a change in power (μJ sec−1) supplied to maintain identical temperatures in the two cells. The data in the curve were used to determine the ΔQws as described in the Materials and Methods. Assuming linearity of ΔQws with change in pressure of 3 bar, the ΔQws/P was determined, and from the slope the Δ(∂CP/∂P)T was calculated. Note, the ΔQws/P and Δ(∂CP/∂P)T used in this manuscript are for a 0.3 mL sample and reference cells. The sample cell contained 20 mg g−1 of the different solutes dissolved in water and the reference cell contained pure water. Examples of the curves for 20 mg g−1 urea and 20 mg g−1 isopropanol are show in Figs. 1 and 2, respectively. The ΔQws/P and Δ(∂CP/∂P)T were plotted against the sum of potential proton acceptors and donors per gram of the solute or co-solvent (calculated using Supplementary Table 1).
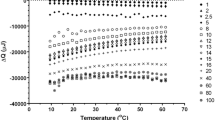
A diverse range of solutes and cosolvents were dissolved in high purity water; the alcohols methanol, ethanol, and isopropanol; the polyols glucose, sucrose, trehalose, mannitol, sorbitol, and glycerol; the carboxylic acids acetic acid, succinic acid and citric acid; acetone and urea. The ΔQws/P at 25 °C for the alcohols, polyols, carboxylic acids, acetone and urea are shown in Fig. 3 and Supplementary Table 2. There is observable clustering of similar organic solute’s ΔQws/P values; the alcohols having a positive ΔQws/P, the polyols with weak negative ΔQws/P, and the carboxylic acids, acetone and urea with strong negative ΔQws/P. There is not an obvious correlation between the ΔQws/P values of the different organic solutes and the hydrogen bonding capacity of the solute (sum of potential proton acceptors and donors per gram of the solute). The Δ(∂CP/∂P)T for the alcohols, polyols, carboxylic acids, acetone and urea are shown in Fig. 4 and Supplementary Table 2. Alcohols have negative Δ(∂CP/∂P)T values, and the polyols, carboxylic acids, acetone and urea have positive Δ(∂CP/∂P)T values. The Δ(∂CP/∂P)T of the alcohols have an apparent correlation with the sum of potential protons acceptors and donors per gram of the solute or co-solvent. The polyols, carboxylic acids, acetone and urea also have an apparent correlation with the sum of potential proton acceptors and donors per gram of the solute or co-solvent. The alcohols are not aligned with the other molecules tested.
ΔQws/P for a range of solutes and solvents in water adjusted to take water displacement into account for the change in pressure from 1 to 4 bar, measured at 25 °C using pressure perturbation calorimetry plotted against the sum of potential proton acceptors and donors per gram of the solute or co-solvent. Polyols (x), amino acids ( +), carboxylic acids (●), acetone (▲), urea (▼) and alcohols (o). All solutes were 2 mass/% in water and the cell volume 0.3 mL
Δ(∂CP/∂P)T for a range of solutes and solvents in water adjusted to take water displacement into account for the change in pressure from 1 to 4 bar, measured at 25 °C using pressure perturbation calorimetry plotted against the sum of potential protons acceptors and donors per gram of the solute or co-solvent. Polyols (x), amino acids ( +), carboxylic acids (●), acetone (▲), urea (▼) and alcohols (o). All solutes were 2 mass/% in water and the cell volume 0.3 mL
The amino acids glycine, serine, proline, and valine were tested in their neutral zwitterionic form. The ΔQws/P for zwitterionic and charged amino acids are shown in Fig. 3 and Supplementary Table 2. The ΔQws/P at 25 °C for both zwitterionic and charged amino acids loosely clustered together with overlap with the polyols and carboxylic acids. The ΔQws/P at 25 °C are not directly related to the sum of potential proton acceptors and donors per gram of the solute but there is a general trend that amino acids with a higher sum of potential proton acceptors and donors per gram of the solute value had weakly negative ΔQws/P and a lower the sum of potential proton acceptors and donors per gram of the solute had a weak negative ΔQws/P. PPC analysis of the zwitterionic amino acids glycine, proline, serine and valine observed positive Δ(∂CP/∂P)T values that are slightly higher than the neutral polyols and carboxylic acids (Fig. 4).
Binary acetone, acetic acid and ethanol aqueous mixtures
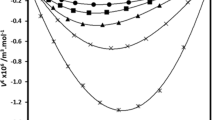
The ΔQws/P measured for ethanol water mixtures with a pressure rise from 1 to 4 bar at 25 °C was plotted against the mole fraction (x2) of the solute, ethanol (Supplementary Fig. 1). This showed a decline in ΔQws/P relative to pure water which leveled out as the proportion of ethanol increased. The Δ(∂CP/∂P)T value for ethanol water mixtures with a pressure rise from 1 to 4 bar at 15 to 35 °C plotted against x2 of ethanol (Fig. 5). The relationship between Δ(∂CP/∂P)T and ethanol x2 had three distinct phases. The Δ(∂CP/∂P)T relative to pure water initially dropped and was negative for the x2 between 0.01 and 0.05, then rose reaching a maximum around x2 of 0.25, then was relatively level at x2 over 0.25. A similar complex relationship between the x2 of ethanol and Δ(∂CP/∂P)T has been observed for isopropanol [29].
The ΔQws/P measured for acetic acid water mixtures with a pressure rise from 1 to 4 bar at 25 °C was plotted against the x2 of acetic acid (Supplementary Fig. 2). It showed a decline in ΔQws/P relative to pure water which leveled out as the proportion of acetic acid increased. The decline in ΔQws/P relative to pure water was not identical to acetone but followed a similar trend. The Δ(∂CP/∂P)T value for acetic acid water mixtures with a pressure rise from 1 to 4 bar, for the temperature range of 15–35 °C plotted against x2 (Fig. 6). Unlike acetone, the relationship between Δ(∂CP/∂P)T and acetic acid x2 was not composed of two linear lines but was curved.
The ΔQws/P measured for acetone water mixtures with a pressure rise from 1 to 4 bar at 25 °C was plotted against the x2 of acetone (Supplementary Fig. 3). It showed a decline in ΔQws/P relative to pure water which leveled out as the proportion of acetone increased. The Δ(∂CP/∂P)T value for acetone water mixtures with a pressure rise from 1 to 4 bar, for the temperature range of 15–35 °C plotted against x2 (Fig. 7). The change in Δ(∂CP/∂P)T between x2 of 0 and 0.08 was proportional to the x2. There was an apparent inflection between x2 of 0.08 and 0.010 where the Δ(∂CP/∂P)T leveled out.
Discussion
Duality of water
Loren Hepler in 1969 wrote “Possibly the simplest useful description of liquid water, is that in which the water is pictured as being a mixture of two species; one a bulky ice-like species with relatively low density and the other a denser species that is formed by breaking (or bending) the hydrogen bonds that maintain the ice-likeness of the bulky species” [15]. Assuming that the Δ(∂CP/∂P)T is dictated by the change to the structure of the water, as suggested by Loren Hepler, and is not directly affected by changes to the structure of the solute. The Δ(∂CP/∂P)T value measured by PPC is a direct measure of water structure. There are likely to be gradients in the perturbation of the structure of water away from the solute, which in turn is affected by the solute concentration [31]. This may be a complex function with contributions from the adoption of low energy dipole orientations as well as a change in hydrogen bond population. PPC enables the structure of water in the presence of a solute to be studied.
Low concentrations of organic solutes in water
The PPC data presented in this paper is consistent with organic solutes having three key effects on water structure.
The first interaction that is observed is between water and hydrophobic alkyl moieties, where the water at the interface forms a less dense structure with a higher hydrogen bond population than bulk water, which is observed as a negative Δ(∂CP/∂P)T. This is seen with methanol, ethanol and isopropanol (Fig. 4). Debate on the nature of water around non-polar molecules or moieties has a long and colorful history. Based on partial molal entropy determined for dilute alcohol and amine solutions, the water around non-polar parts of polar molecules were described as frozen patches or mini-icebergs [32]. Another explanation was that this water more closely resembled the crystalline hydrates that form around methane, ethane and propane in water. These clathrate-like cages are structurally different to ice [33]. More recent work using femtosecond midinfrared spectroscopy to study the orientational dynamics of water has shown that non-polar groups are surrounded by a number of water molecules that display much slower orientational dynamics than the bulk water with four immobilized water molecules around a methyl group [34]. These changes are seen in terms of orientational structures near hydrophobic interfaces, with perturbations statistically significant over a few nm’s [35]. It is worth noting that the number of immobilized water molecules is partially determined by the sensitivity of the analytical technique used. The structuring of water around hydrophobic solutes (methane, ethane, krypton, and xenon) studied using infrared spectroscopy and MD simulation suggested that water molecules form stronger hydrogen bond with each other when not surrounded by other water molecules, that it is the absence of water molecules due to the presence of the hydrophobic molecule that enables the formation of stronger hydrogen bonds [36, 37]. It was also suggested that different hydrophobic solutes or moieties will have varying influence on the local ordering of water molecules, making generic assumptions of the exact structure of the water problematic [38]. The term clathrate-like cages will be applied to the water in the presence of the hydrophobic moieties in this paper.
The second interaction is due to the solute hydrogen bonding with water, interfering with water’s opportunity to form tetrahedral ice-like water, observed as a positive Δ(∂CP/∂P)T. There is an apparent relationship between the proton donating or accepting capacity of the solutes (acetone, the polyols, the carboxylic acids and urea) and the disruption of the water structure (Fig. 4).
The third interaction between charged or zwitterionic solutes is Coulombic. Previous research has observed a positive Δ(∂CP/∂P)T associated with inorganic salts [4, 39]. The interaction between water and charged solutes has been widely studied. The phenomenon known as electrostriction, where the electrical field associated with a changed solute causes a reduction of the apparent partial volume of the solute due to a reduction in the volume of the surrounding water and loss of water structure [12]. The zwitterionic amino acids glycine, proline, serine and valine had positive Δ(∂CP/∂P)T values that close to the polyols and carboxylic acids suggesting that Coulombic force played a negligible part in altering the structure of water (Fig. 4).
Binary ethanol–water mixtures
Ethanol has an ethyl and a hydroxyl moiety. It can act as a proton donor or acceptor and is able to hydrogen bond with itself. The alcohols have an amphiphilic structure and while miscible in water, they do segregate into alcohol and water clusters or aggregates on the micro-scale. Assuming the hypothesis that Δ(∂CP/∂P)T is a measure of the structure of water holds for solutions with higher mole fraction (x2) of the solute, then the behavior of the ethanol–water mixture can be studied from the perspective of the structure of the water component. The negative Δ(∂CP/∂P)T values observed at relatively low concentrations (x2 < 0.07) in Fig. 5, are consistent with the presence of a more highly hydrogen-bonded population than is present in bulk water, which could be due to water forming “clathrate-like” cages around ethanol’s ethyl moiety. The zero Δ(∂CP/∂P)T value observed around x2 ≈ 0.07 may correspond to increasing interaction between ethanol’s ethyl groups, displacing the water associated with the “clathrate-like” cages. Between 0.07 < x2 < 0.25, the presence of ethanol molecules increasingly disrupts the “ice-like” structure in the remaining water fraction. By x2 ≈ 0.25 the population of “ice-like” water in the remaining water fraction was gone.
Ethanol–water mixtures have been subject to extensive experimentation for over 50 years. It is known to exhibit non-ideal behavior in aqueous solutions. Ethanol–water mixtures have been studied using a diverse array of techniques including calorimetric studies of ethanol–water mixing [40, 41], Raman and FTIR spectroscopy [42,43,44]; Terahertz spectroscopy [45]; X-ray diffraction and soft X-ray adsorption [46, 47]; adiabatic compressibility [48], and nuclear magnetic resonance [45, 49] providing a range of different perspectives on the phenomenon.
Adiabatic compressibility calculated from the sound velocity of ethanol–water mixtures observed two transitions at mole fractions, x2.1 0.060 ± 0.002 and x2.2 at 0.29 ± 0.03, which match the transitions observed in the PPC data [48]. The adiabatic compressibility results were interpreted as simultaneous existence of two independent interactions between the solute and water; firstly, clathrate-like partial cage structures associated with the ethanol’s ethyl group and secondly, the hydrogen bond formed between the ethanol’s hydroxyl and water. They also linked the hydrogen bond formation with the ethanol hydroxyl group with the formation of a less compressible water structure. At x2 < x2.1 hydration of the ethyl group dominates the change in compressibility where at x2 < x2.2 self-association between the ethyl groups is at the expense of the cage structures. The authors then attribute the change in compressibility at x2 > x2.2 to the interaction between water and the ethanol’s hydroxyl group though they don’t link this to “ice-like” water. The difference in interpretation is that at x2 < x2.1 hydration of the ethyl group dominates the change in compressibility but that self-association between the ethyl groups starts at very low ethanol concentrations and eliminates the clathrate-like cage structures by x2 = x2.1 and at x2.1 < x2 < x2.2 the ongoing interaction between water and the ethanol’s hydroxyl group interferes with the formation of “ice-like” water. The formation of micelle-like microaggregates at x2 > x2.2 is observed indirectly by its effect on clathrate-like and ice-like water structure.
Raman spectroscopy of ethanol–water mixtures using the O–H stretching band (3050–3700 cm−1) can be deconvoluted to reveal a strongly H-bonded hydroxyl group around 3200 cm−1, a weakly H-bonded hydroxyl group around 3450 cm−1 and bond free hydroxyl groups at 3650 cm−1. By dividing the intensity at 3200 cm−1 by 3420 cm−1 the proportion of strongly bonded hydroxyl groups can be estimated. A peak in hydrogen bond strength was observed around x2 = 0.04 and 0.09 that was interpreted as ethanol hydrates similar to gaseous ethanol clathrates [42]. At x2 between 0.09 and 0.35, the hydrogen bond strength declined which was attributed to the loss of the ethanol hydrates and a loss of structure in the water not directly associated with the ethyl moieties. The fact the decline continued between x2 values of 0.25 and 0.35 suggests ethanol continued to weaken water’s hydrogen bonds after the “ice-like” water population had been depleted suggesting that PPC is only seeing part of the picture during the complex interaction between ethanol and water.
Pulse gradient spin-echo (PGSE) NMR analysis of ethanol–water mixture diffusion coefficients was also consistent with clathrate-like hydrate formation at x2 = 0.06 [50]. Terahertz spectroscopy measurement of absorption coefficients of ethanol–water mixtures was also consistent with ethanol causing disruption of water structure between x2 = 0.20 and 0.30 [45].
Ethanol–water mixtures present a complex picture with the behavior at low concentrations dominated by the formation of clathrate-like cages around ethyl groups, as the concentration increased micelle-like clustering of the ethyl groups occurs eventually forming extended hydrogen-bonded networks which change their behavior as the ethanol composition increased. PPC provides evidence on this behavior purely from the perspective of the structure of the water component in the mixture.
Binary acetic acid–water mixtures
Acetic acid has a methyl and a carboxyl moiety. The carbonyl group can act as a proton acceptor or donor and can form pairs in its liquid form and in the gas-phase. Acetic acid can also form single hydrogen bonds with other acetic acids to form chains or similar structures. The PPC did not detect any negative Δ(∂CP/∂P)T values at low acetic acid concentrations which would have been indicative of clathrate-like cages around methyl group. The curvature in the Δ(∂CP/∂P)T value versus \({x}_{2}\) suggests the acetic acid is not just hydrogen bonding with water but has competing self-association which is either a mixture of dimer formation or single hydrogen bonds to form dimers or longer chains. There was no direct evidence for micelle-type interactions between the methyl groups of the acetic acid molecules though this cannot be discounted. Like ethanol, acetic acid is known to exhibit non-ideal behavior in aqueous solutions.
Raman spectroscopy of the acetic acid C=O stretching vibrational band shifted from 1665 cm−1 to the weaker 1715 cm−1 when acetic acid was added to water. This suggests the dimer configuration seen in liquid acetic acid is broken in water [51]. Dimer formation measured using anisotropic optical Kerr effect spectroscopy showed cyclic dimer is the predominant dimer but that at low concentrations x2 < 0.3 the quantity of dimer was low [52].
Raman spectroscopy of the water O–H stretching vibrational modes demonstrated a decline in strongly bonded water with an increase in weakly bonded water [51]. When the intensity ration of strongly bonded water to the weakly bonded water was plotted against mole fraction, the loss of strongly bonded water is comparable with the rise in Δ(∂CP/∂P)T seen in Fig. 6. The Raman with the PPC data provided evidence that acetic acid breaks the hydrogen bond networks of water. Refractive index of acetic acid–water mixtures has a maximum value at a concentration around x2 = 0.3 [53]. This was interpreted as a change in the dipole moments of water molecules in a way that they could not contribute to the dielectric polarization of the mixture, which is typically influenced by cooperative local dipole moment orientations (the Kirkwood g-factor) [54].
The self-association between acetic acid molecules is more complex than an equilibrium between monomeric and dimeric acetic acid configurations but could include heteromolecular structures which incorporated hydrogen-bonding events between multiple acetic acid and water molecules. The similarity of the estimation in hydrogen-bonded structure of water by Raman spectroscopy and the Δ(∂CP/∂P)T measured by PPC at different mole fractions of acetic acid is evidence that the hypothesis that Δ(∂CP/∂P)T is a measure of water structure is true for acetic acid water mixtures.
Binary acetone–water mixtures
Acetone has two methyl and a carbonyl moiety. The carbonyl group can act as a proton acceptor but not a donor so it cannot hydrogen bond with itself. The Δ(∂CP/∂P)T for acetone–water mixtures measured by PPC observed as a linear rise in Δ(∂CP/∂P)T between x2 = 0.00 and 0.10 followed by a plateauing of the Δ(∂CP/∂P)T value at x2 > 0.10 (Fig. 7). There is an obvious transition around x2 = 0.10. This is consistent with addition acetone interfering with the “ice-like” structure of water, a process that is complete at x2 = 0.10. Linearity in the Δ(∂CP/∂P)T value versus \({x}_{2}\) suggests the acetone is only hydrogen bonding with water and is not forming any complex hydrogen-bonded structures. There was no evidence of negative Δ(∂CP/∂P)T at low acetone concentrations that would be indicative of clathrate-like cage around methyl groups. There was also no direct evidence for micelle-type interactions between the methyl groups though cannot be discounted.
The formation of a 1:1 complex of acetone and water linked by a hydrogen bond between the water hydroxyl and the acetone carbonyl group has been observed using FT-infrared absorption spectroscopy which is consistent with the chemical structure of acetone [55].
Low frequency Raman spectroscopy of acetone–water mixtures studied two distinct translational modes [56]. The peak at 60 cm−1 is attributed to motion perpendicular to the O–H…H direction, bending the hydrogen bond. The frequency of this band rose rapidly as the acetone concentration increased to x2 = 0.06 then slowly rose to a peak at x2 = 0.14, then slowly declined. The rise in frequency was attributed to stiffening of the molecular cage around the water. The peak at 190 cm−1 is for motion along the hydrogen bond axis. The frequency monotonically decreases with increased acetone mole fraction. This was attributed to a slow decline in the structure of water. This interpretation of the Raman spectroscopy data is not consistent with the PPC data.
The linearity in the Δ(∂CP/∂P)T versus x2 value with a transition around x2 = 0.10 is unique to PCC data. This supports the conclusion that the relationship between acetone and water at different concentrations is complicated and would require an orthogonal approach to fully understand this binary mixture.
Conclusions
The PPC data are consistent with the hypothesis that water has a dual nature, with a less dense highly hydrogen-bonded “ice-like” structure and a denser less hydrogen-bonded state. That following Le Chatelier’s principle, the application of pressure to water will result in the water population shifting from the less dense to the denser state with resulting breakage of hydrogen bonds and a change in heat (ΔQws), which is measured by the calorimeter. The calorimeter measures the difference between the sample and pure water in the reference cell. This enables the Δ(∂Qws/∂P)T and the Δ(∂CP/∂P)T to be calculated. The authors did not use the partial specific volume of the solute in their calculations as the value is erroneous at higher solute concentrations due to solute self-association or overlapping hydration layers. The authors content that the logic formulated by Loren Hepler that Δ(∂CP/∂P)T is a measure of the structure of water is correct and holds for the solute concentrations presented in this paper.
That at low solute concentrations, it is the effect of the solute on the structure of water that is being measured by the PPC. That at low solute concentrations, the alcohols demonstrate “clathrate” type behavior increasing the populations of structured water. That most solutes interfere with waters ability to form “ice-like” structured water and this is often due the solute hydrogen bonding with the water. Solute self-association mediated by hydrogen bonding can be observed as curvature in the Δ(∂CP/∂P)T versus mole fraction relationship as solute self-association competes with cosolute–water hydrogen bonding. That solute self-association mediated by hydrophobic interaction, including micelle-like structures or mesoscopic networks, are not measured by PPC unless the interaction indirectly interferes with the formation of “ice-like” water. This is based only on hydrogen bonding considerations to the PPC measurements (i.e., contributions from more complex dispersion forces are not considered).
To get a complete picture of what is happening in binary mixtures a combination of analytical techniques should be used. What PPC provides for the study of binary mixtures is an understanding of the solutes effect on the structure of the water component, which is an important aspect of the binary mixture’s behavior. For ethanol–water binary mixtures (Fig. 5), the PPC data demonstrated a level of complexity in the solution that is missed by many other techniques [45]. Conversely, for acetone–water binary mixtures, the PPC data are clear but when compared to the excess enthalpy data and the Raman shift it suggests that PPC is only seeing part of the picture.
To date, PPCs greatest contribution to solution chemistry was the work disproving the hypothesis that solutes affect protein stability indirectly by making or breaking water structure [4]. What is missing is research using PPC to better understand the role of water structure in determining the interactions between macromolecules and solutes. There are hypotheses that explain water mediated modulation of macromolecule stability and solubility in terms of “preferential hydration” [57] or interfacial surface tension [58] where PPC could be applied in conjunction with spectroscopic and computational approaches to better understand these systems [59, 60].
References
Plotnikov VV, Brandts JM, Lin L-N, Brandts JFA. New ultrasensitive scanning calorimeter. Anal Biochem. 1997;250:237–44.
Jelesarov I, Bosshard HR. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J Mol Recognit. 1999;12:3–18.
Cooper A, Johnson CM, Lakey JH, Nöllmann M. Heat does not come in different colours: entropy–enthalpy compensation, free energy windows, quantum confinement, pressure perturbation calorimetry, solvation and the multiple causes of heat capacity effects in biomolecular interactions. Biophys Chem. 2001;93:215–30.
Batchelor JD, Olteanu A, Tripathy A, Pielak GJ. Impact of protein denaturants and stabilizers on water structure. J Am Chem Soc. 2004;126:1958–61.
Röntgen WC. VIII Ueber die constitution des flüssigen wassers. Ann Phys. 1892;45:91–7.
Frank HS, Wen W-Y. III Ion-solvent interaction. Structural aspects of ion-solvent interaction in aqueous solutions: a suggested picture of water structure. Discuss Faraday Soc. 1957;24:133–40.
Bernal JD, Fowler RH. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J Chem Phys. 1933;1:515.
Head-Gordan T, Johnson ME. Tetrahedral structure or chains for liquid water. Proc Natl Acad Sci USA. 2006;103:7973–8797.
Ball P. Water—an enduring mystery. Nature. 2008;452:291–2.
Tokushima T, Harada Y, Takahashi O, Senba Y, Ohashi H, Pettersson LGM, Nilsson A, Shin S. High-resolution X-ray emission spectroscopy of liquid water: the observation of two structural motifs. Chem Phys Lett. 2008;460:387–400.
Nilsson A, Petterson LGM. The structural origin of anomalous properties of liquid water. Nat Com. 2015;6:8998.
Cox WM, Wolfenden JH. The viscosity of strong electrolytes measured by a differential method. Proc R Soc London Ser A. 1934;92:475–88.
Von Hippel PH, Wong K-Y. On the conformational stability of globular proteins. J Biol Chem. 1965;240:3909–23.
Washabaugh MW, Collins KD. The systematic characterisation by aqueous column chromatography of solutes which affect protein stability. J Biol Chem. 1986;261:12477–85.
Hepler L. Thermal expansion and structure in water and aqueous solutions. Can J Chem. 1969;7:4613–7.
Lin LN, Brandts JF, Brandts JM, Plotnikov V. Determination of the volumetric properties of proteins and other solutes using pressure perturbation calorimetry. Anal Biochem. 2002;302:144–60.
Ravindra R, Winter R. Pressure perturbation calorimetry: a new technique provides surprising results on the effects of co-solvents on protein solvation and unfolding behaviour. ChemPhysChem. 2004;5:566–71.
Kamerzell TJ, Ramsey JD, Middaugh CR. Immunoglobulin dynamics, conformational fluctuations, and nonlinear elasticity and their effects on stability. J Phys Chem B. 2008;112:3240–50.
Zhai Y, Okoro L, Cooper A, Winter R. Applications of pressure perturbation calorimetry in biophysical studies. Biophys Chem. 2011;156:13–23.
Pandharipande PP, Makhatadze GI. Thermal expansivities of peptides, polypeptides and proteins as measured by pressure perturbation calorimetry. Methods. 2015;76:61–6.
Suladze S, Kahse M, Erwin N, Tomazic D, Winter R. Probing volumetric properties of biomolecular systems by pressure perturbation calorimetry (PPC)—the effects of hydration, cosolvents and crowding. Methods. 2016;76:67–77.
Dragan AI, Russell DJ, Privalov PL. DNA hydration studies by pressure perturbation scanning microcalorimetry. Biopolymers. 2009;91:95–101.
Makhatadze GI, Chen CR, Khutsishvili I, Marky LA. The volume changes of unfolding of dsDNA. Biophys J. 2022;121:4892–9.
Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–701.
Heerklotz H. The microcalorimetry of lipid membranes. J Phys Condens Mat. 2004;16:R441–67.
Nazari M, Fan HY, Heerklotz H. Effect of hydrophobic interactions on volume and thermal expansivity as derived from micelle formation. Langmuir. 2012;28:14129–36.
Kujawa P, Winnik FM. Volumetric studies of aqueous polymer solutions using pressure perturbation calorimetry: a new look at the temperature-induced phase transition of poly(N-isopropylacrylamide) in water and D2O. Macromolecules. 2001;34:4130–5.
Aseyev V, Hietala S, Laukkanen A, Nuopponen M, Confortini O, Du Prez FE, Tenhu H. Mesoglobules of thermoresponsive polymers in dilute aqueous solutions above the LCST. Polymer. 2005;46:7118–31.
The Engineering ToolBox. Volumetric (Cubic) thermal expansion, 2009. https://www.engineeringtoolbox.com/volumetric-temperature-expansion-d_315.html. Accessed 21 Sept 2023.
Bye JW, Freeman CL, Howard JD, Herz G, McGregor J, Falconer RJ. Analysis of mesoscopic structured 2-propanol/water mixtures using pressure perturbation calorimetry and molecular dynamic simulation. J Sol Chem. 2017;46:175–89.
Besford QA, Van den Heuvel W, Christofferson AJ. Dipolar dispersion forces in water–methanol mixtures: enhancement of water interactions upon dilution drives self-association. J Phys Chem B. 2022;126:6231–9.
Frank HS, Evans MW. Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; partial molal entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J Chem Phys. 1945;13:507–32.
Kauzmann W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63.
Rezus YLA, Bakker HJ. Observation of immobilised water molecules around hydrophobic groups. Phys Rev Lett. 2007;99: 148301.
Besford QA, Christofferson AJ, Kalayan J, Sommer J-U, Henchman RH. The attraction of water for itself at hydrophobic quartz interfaces. J Phys Chem B. 2020;124:6369–75.
Grdadolnik J, Merzel F, Avbelj F. Origin of hydrophobicity and enhanced water hydrogen bond strength near purely hydrophobic solutes. Proc Natl Acad Sci USA. 2017;114:322–7.
Merzel F, Avbelj F. Why do water molecules around small hydrophobic solutes form stronger hydrogen bonds than in the bulk? BBA Gen Sub. 2020;1864: 129537.
Grabowska J, Kuffel A, Zielkiewicz J. Revealing the Frank−Evans “Iceberg” structures within the solvation layer around hydrophobic solutes. J Phys Chem B. 2021;125:1611–7.
Bye JW, Falconer RJ. A study of the relationship between water and anions of the Hofmeister series using pressure perturbation calorimetry. Phys Chem Chem Phys. 2015;17:14130–7.
Franks F, Ives DJC. The structural properties of alcohol–water mixtures. Q Rev Chem Soc. 1966;20:1–44.
Boyne JA, Williamson AG. Enthalpies of mixing of ethanol and water at 25 °C. J Chem Eng Data. 1967;12:318.
Burikov S, Dolenko T, Patsaeva S, Starokurov Y, Yuzhakov V. Raman and IR spectroscopy research on hydrogen bonding in water–ethanol systems. Mol Phys. 2010;108:2427–36.
Nishi N, Takahashi S, Matsumoto M, Tanaka A, Muraya K, Takamuku T, Yamaguchi T. Hydrogen bonding cluster formation and hydrophobic solute association in aqueous solution of ethanol. J Phys Chem. 1995;99:462–8.
Lin K, Hu N, Zhou X, Liu S, Luo Y. Reorientation dynamics in liquid alcohols from Raman spectroscopy. J Raman Spectrosc. 2012;43:82–8.
Li R, D’Agostino C, McGregor J, Mantle MD, Zeitler JA, Gladden LF. Mesoscopic structuring and dynamics of alcohol/water solutions probed by terahertz time-domain spectroscopy and pulsed field gradient nuclear magnetic resonance. J Phys Chem B. 2014;118:10156–66.
Nagasake M, Bouvier M, Yuzawa H, Kosugi N. Hydrophobic cluster formation in aqueous ethanol solutions probed by soft X-ray absorption spectroscopy. J Phys Chem B. 2022;126:4948–55.
Juurinen I, Pylkkanen T, Sahle CJ, Simonelli L, Hämäläinen K, Huotari S, Hakala M. Effect of the hydrophobic alcohol chain length on the hydrogen-bond network of water. J Phys Chem B. 2014;118:8750–5.
Omori G, Santucci A. Dynamical and structural properties of water/alcohol mixtures. J Mol Liq. 1992;69:161–81.
Coccia A, Indovina PL, Podo F, Viti V. PMR studies on the structures of water−ethyl alcohol mixtures. Chem Phys. 1975;7:30–40.
Price WS, Ide H, Arata Y. Solution dynamics in aqueous monohydric alcohol systems. J Phys Chem A. 2003;107:4784–9.
Nishi N, Nakabatashi T, Kosugi K. Raman spectroscopic study on acetic acid clusters in aqueous solutions: dominance of acid–acid association producing microphase. J Phys Chem A. 1999;103:10851–8.
Heisler IA, Mazur K, Yamaguchi S, Tominaga K, Meech SR. Measuring acetic acid dimer modes by ultrafast time-domain Raman spectroscopy. Phys Chem Chem Phys. 2011;13:15573–9.
Gofurov S, Makhmanov U, Kokhkharov A, Ismailova OB. Structural and optical characteristics of aqueous solutions of acetic acid. Appl Spectrosc. 2019;73:503–10.
Besford QA, Christofferson AJ, Liu M, Yarovsky I. Long-range dipolar order and dispersion forces in polar liquids. J Chem Phys. 2017;147: 194503.
Zhang KK, Lewars EG, March RE, Parnis JM. Vibrational spectrum of the acetone-water complex: a matrix isolation FTIR and theoretical study. J Phys Chem. 1993;97:4320–5.
Idrissi A, Longelin S, Sokolić F. Study of aqueous acetone solution at various concentrations: low-frequency Raman and molecular dynamics simulations. J Phys Chem B. 2001;105:6004–9.
Timasheff SN. Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry. 2002;91:13473–82.
Zhang Y, Cremer PS. The inverse and direct Hofmeister series for lysozyme. P Natl Acad Sci USA. 2007;106:15249–53.
Gibb BC. Hofmeister’s curse. Nat Chem. 2019;11:963–5.
Rogers BA, Okur HI, Yan C, Yang T, Heyda J, Cremer PS. Weakly hydrated anions bind to polymers but not monomers in aqueous solutions. Nat Chem. 2022;14:40–5.
Acknowledgements
The authors would like to thank the University of Adelaide for their financing the purchase of the differential scanning calorimeter used in this work.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
LTB and QAB contributed to the interpretation of data, drafting of the work, and ensuring the intellectual content. RJF contributed to the experimental design, data collection, mathematical analysis, composition of the first draft of the manuscript, and its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toronjo-Urquiza, L., Besford, Q.A. & Falconer, R.J. Interactions between small organic molecules and water measured using pressure perturbation calorimetry. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13443-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13443-7