Abstract
The thermally activated clay minerals are well-known as perspective supplementary cementing materials (SCMs) able to reduce the Portland clinker consumption and thus also the related CO2 emissions. The best SCM performance among clay minerals is provided by thermally activated kaolin (metakaolin). Nevertheless, kaolinitic clays are not available everywhere, while varying mixed clays can be considered as globally affordable raw materials. The present work deals with the thermal activation of four illitic-kaolinitic clays with varied content of clay minerals, quartz and calcite, available locally in the Czech Republic. The attainment of activation was evaluated with the help of mechanical strength and the saturated lime test. There is no doubt that kaolin is the best clay for SCM production. However, the activation of mixed illitic-kaolinitic clays at 600 °C provided comparable and sufficient performance, despite that the crystal structure of illite was not completely decomposed. The non-clay minerals presence did not reduce the activity either; even the clay containing just about 50% of clay minerals (mostly illite) treated at 650 °C provided sufficient mechanical performance. As the drawback of activated illitic clays must be considered their higher sensitivity to the proper calcination temperature compared to metakaolin since illite (and other 2:1 clay minerals) does not form any metastable dehydroxylated phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reduction of CO2 emissions related to concrete, and specifically to Portland cement production, is nowadays a huge challenge for the construction industry. There is a number of approaches how to reduce the negative environmental effects of Portland cement production; it may be reached either by the increasing energy efficiency of the current Portland clinker process [1] or by an alternative, and possibly more sustainable, materials on a different chemical basis such are, e.g. belite-ye'elimite-ferrite (BYF) clinkers [2] or geopolymers [3]. The substitution of part of the Portland clinker by a supplementary cementing material (SCM) may be considered as a well-known and conventional approach to the reduction of Portland clinker consumption, but there is still very intensive research in this field [4]. Conventional SCMs like coal fly ash and ground granulated blast furnace slag are not (and will not be in future) able to satisfy the demand of the cement industry thus new SCMs are searched. Probably, the most innovative and promising—both technically and environmentally—today's approach is called LC3 cement, i.e. “limestone calcined clay cement” [5]. In this type of cement, the content of clinker is reduced to 50%, while the rest of the material is limestone (15%), gypsum (5%) and calcined clay (30%) in the role of pozzolanic component. Briefly, the pozzolanic activity means the ability of an aluminosilicate material to react with Ca(OH)2 and form C–S–H products. In the case of calcined clays, the pozzolanic activity originates in the thermal dehydroxylation of clay minerals, which is resulting in the destruction of the crystalline structure to a more or less amorphous matter. Very important is the proper calcination/activation temperature since the clay dehydroxylation is followed by sintering and crystallization of new minerals, what is reducing the pozzolanic activity [6]. So far the highest attention has been paid to calcined kaolinitic clay as the LC3 component since calcined kaolinite (or more generally 1:1 clay minerals) provides the highest pozzolanic activity among the clay minerals [7]. On the other hand, pure kaolinites are relatively valuable raw materials while the low-grade clays, often containing more 2:1 clay minerals (and a range of non-clay minerals), are easily available all around the world. The results of Schulze and Rickert [8] showed that a broad range of calcined clays, including chloritic clays and bentonites, provides sufficient pozzolanic activity in terms of European standard EN 197–1. It has to be noticed that calcined clays may be obviously used also as precursors in alkaline activation [9].
With respect to local availability and sustainability, the utilization of low-grade mixed clay as a source of SCM is favourable compared to more costly and less abundant “pure” clays applicable for ceramics production or even more advanced applications [10]. Such low-grade clay sources suitable for application as SCM may be properly calcined mine tailings [11], dredged sediments from rivers and lakes [12] or coal gangue [13]. For these materials, the presence of more clay minerals and also some other impurities such are quartz, calcite and other carbonates is typical [8]. Organic matter may be also found, especially in sediments [14].
As it was already mentioned above, the thermal activation of clays lies in the dehydration and dehydroxylation of clay minerals. These processes were primarily studied by thermal analysis techniques since the thermal response of clay minerals is served as one of the fundamental tools for clay minerals identification [15]. Since the main traditional application field of clay minerals has been ceramics production, the dehydroxylation and sintering of especially kaolinite has been widely studied [16]. Finally, in the last years, the thermal decomposition of clay minerals is widely studied with respect to the thermal activation to acquire the highest pozzolanic activity. These studies have been performed both on pure or mixed clays [14, 17]. The present study deals with four industrially (i.e. on a large scale) available mixed kaolinitic-illitic clays which differ in mineralogical composition; their phase composition covers a continuous range from highly kaolinitic clay to a brick soil containing just a few % of kaolinite (and high amount of quartz and calcite). The clays were thermally activated at different temperatures in order to identify the most suitable temperature for the activation. The aim of the study was also to evaluate the influence of “impurities” on the thermal activation kinetics and on the resulting pozzolanic activity.
Experimental
Experimental methods
The chemical composition of raw clays was examined by ED XRF spectrometer Quant´X (Thermo). The content of H2O and CO2 was estimated with the help of thermogravimetry. The simultaneous TG/DSC analysis of clays was performed by Setaram Labsys Evo; the sample was placed in an alumina crucible and after temperature stabilization (at 30 °C), the temperature increased with a specific heating rate up to reaching 1000 °C. The experiments were performed in an argon atmosphere with a flow rate of 40 mL min−1. The phase composition of clays was studied by X-ray diffraction (XRD); diffractograms were recorded using Malvern PANalytical Aeris diffractometer equipped with a CoKα source operating at 7.5 mA and 40 kV. The incident beam path consisted of an iron beta-filter, Soller slits 0.04 rad and divergence slit 1/2°. The diffracted beam path was equipped with 9 mm anti-scatter slit and Soller slits 0.04 rad. The used detector was PIXcel1D-Medipix3 detector with an active length 5.542°. Data were evaluated by Rietveld refinement performed by Profex software (ver. 5.0.2) [18]. The phase composition of activated clays was examined by the device described above but in addition, the content of amorphous matter was determined by the employment of an internal standard (20% of ZnO was used). The morphology of clay particles was observed by scanning electron microscope Phenom XL operated in BSE mode, and the particle size distribution was determined by laser diffraction technique, namely Bettersizer device was used.
The influence of calcination temperature on the pozzolanic activity was evaluated by two following methods—the saturated lime test and the mechanical strengths of blended cement paste. The saturated lime test [19] consisted of direct monitoring of Ca2+ binding in the pozzolana sample. The test was carried out by contacting 1 g of pozzolana with 100 mL of saturated Ca(OH)2 solution; the suspension was mixed with an orbital shaker (120 rpm) at 40 °C. The ability of pozzolana to bind Ca2+ ions was evaluated with the help of analysis of the residual solution after the pozzolana was filtered out after 168 h. The residual solution was analysed for OH− and Ca2+ by titration by HCl and EDTA according to standard EN 196–5. The second approach was based on the preparation of blended pastes based on ordinary Portland cement (CEM I 42.5 R) and thermally activated clay (80:20); the water/(cement + pozzolana) ratio was 0.3. The paste was placed in 100 × 20 × 20 mm moulds in order to prevent shrinkage cracks, which could appear, due to the absence of aggregates, if the standard moulds 160 × 40 × 40 mm would be used. Prepared beams were stored in the climatic chamber (20 °C, 98% RH) for 28 days. Afterwards, bending and compressive strength were measured using the loading device FP100 with the Compression Frame Jig Assembly (ELE International). Except for the dimension, the measurement procedure was according to standard EN 1015–11.
Studied materials
Four types of industrially produced mixed clays were studied. Sample K was kaolinitic clay suitable for sanitary ceramics, wall and floor tiles and refractories. Contrary, sample IK was illitic-kaolinitic clay used for tiles production. The sample QIK was similar to IK, but it contained a significantly higher amount of quartz. Finally, CQIK is brick soil containing a very high amount of calcite. Characterization of these raw materials is provided in Results in Chapter 3.1.
Results and discussion
Characterization of raw clays
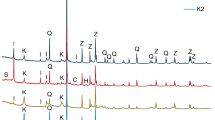
The identification of present minerals in the raw mixed clay was based on powder XRD (Fig. 1). The clay K contains a high amount of kaolinite and a lower amount of illite; quartz is present in very small amount. Sample IK is sort of the opposite case—it contains a higher amount of illite and not negligible amount of quartz and some feldspars (diffractions at about 32°2θ). Material QIK is distinguished by a substantial amount of quartz. Brick soil CQIK contains a significant amount of calcite in addition to the clay minerals (illite + kaolinite) and quartz. Noticeable peaks of gypsum and dolomite were observed as well.
The semi-quantification of the main components of mixed clays was performed with the help of thermogravimetry (Fig. 2). XRD indicated that all materials contain dominantly two clay minerals: kaolinite and illite. Generally, the thermal response of clay minerals consists of dehydration (endothermic removal of interlayer water) and dehydroxylation (endothermic loss of structural OH groups and consequent release of water molecules). Kaolinite is generally supposed to undergo just dehydroxylation [20] even though a certain small amount of water may be sometimes observed to be evolved also between 50 and 200 °C [21]. Nevertheless, for the purpose of quantification of clays’ main components, the relative mass change up to 200 °C was supposed to be just because of the illite dehydration, while the dehydroxylation of both kaolinite and illite contributed to the mass loss between 400 and 700 °C. The DSC signal of CQIK involved a distinct endothermic signal centred at 150 °C, which most likely belonged to small amount of gypsum in this clay (as was is indicated also by elementary composition in Tab. 1). The boundary between dehydroxylation and CaCO3 decomposition in CQIK sample was assumed to be at 650 °C according to DSC (Fig. 2). The phase composition of clays under study (Tab. 2) was thus estimated by help of the TG. The stoichiometric factors of the ongoing processes were assumed as follows [22]: illite dehydration 25; illite dehydroxylation 23.3; kaolinite dehydroxylation 7.2. The column of “quartz” involves all thermally stable minerals (i.e. also feldspars in this case) and column “CaCO3” involves calcite and other forms of calcium carbonate. The XRD indicated just calcite, but the broad endothermic temperature range observed on TG/DSC of CQIK sample (before the peak centred at 863 °C) indicated that also amorphous particles of CaCO3 may be present. The significant endothermic peak at 906 °C (CQIK) was probably caused by the thermal decomposition of CaSO4 impurity to CaO, SO2 and O2 [23]. The DSC curves of samples K and IK contain also an exothermic effect just before 1000 °C. It is caused by crystallization of a new phase, usually called Al-Si spinel (2Al2O3·3SiO2) [16]. It is formed from metakaolinite hence this exothermic effect is pronounced especially in clay K. Finally, the total oxide composition based on XRF and LOI is presented in Tab. 1. It is in obvious agreement with the measured phase composition; the content of potassium clearly corresponds to the illite. SO3 indicates sulphates, most likely a small amount of gypsum. Considering the amount, it was estimated that gypsum content was up to 1.5%. Thus in further discussion, its presence was neglected.
When focused on the particle size distribution (PSD), the d50 of clays K, IK and QIK was well below 10 μm; just the brick soil CQIK was somewhat coarser (Fig. 3). The morphology of raw clays was examined by SEM (Fig. 4). Samples K and QIK were composed of more separated particles, while IK and CQIK had a tendency to clump together. Nevertheless, these clumps have been disintegrated during the PSD measurement by sonication; thus the two methods—PSD and SEM—provided two kinds of information.
Thermal activation of mixed clays
The thermal activation of clays was performed at 500, 550, 600 and 650 °C which covers the range where the clays dehydroxylation was taking place (Fig. 2). It was performed in an electric muffle oven at a given temperature for 3 h with the heating rate of 10 °C min−1. After the heating, the clays were left in the oven for spontaneous cooling. The process of thermal activation was observed by XRD. Figure 5 shows, as an example, the decrease in intensity of diffraction lines of illite (001) and kaolinite (001) with the activation temperature; these data came from the CQIK sample. It is obvious that calcination at 600 °C was able to break down completely the crystalline structure of kaolinite, which is in agreement with the TG/DSC of pure kaolinite published elsewhere [21]. On the other hand, the illite crystal structure was not yet completely destroyed at 650 °C, even though the TG/DSC of the CQIK and also of IK (Fig. 2) indicated that this temperature is sufficient for the illite dehydroxylation. It means that dehydroxylation is not equal to complete breakdown of crystalline lattice. In fact, some residuals of illite structure (001) were detected also in CQIK sample calcined at 1000 °C where new phases (wollastonite CaO·SiO2 and gehlenite Ca2Al[AlSiO7]) were already crystallized (Fig. 4). At this temperature, the illite diffraction 002 already disappeared.
The summary of clays thermal activation is provided in Fig. 6. It has to be kept in mind that the present XRD results were obtained at ambient temperature. The amorphous phase was formed as a result of clay minerals dehydroxylation and collapse of the crystalline structure. The kaolinite decomposition became complete at a lower temperature than illite decomposition, as was already indicated in Fig. 5. The samples K and CQIK provided 001 and 002 diffractions of illite at even 650 °C while the IK and IKQ not. It is likely caused by variability of illite structure and properties, including thermal stability, between individual localities where clays were excavated. Obviously, the quartz and most of the carbonates remained unaffected until 650 °C. The content of quartz in samples somewhat increased with activation temperature due to the fact that the total mass of the sample was lower due to the water escape during the dehydration and dehydroxylation of clay minerals.
The TG/DSC experiment with several heating rates was performed in order to compare the rate of decomposition processes in the clays under the study. The well-known Kissinger method [23] was used for DTG curves even though authors are aware of its shortcomings [24, 25]. The relevant plots are shown in Fig. 7 for dehydration (a) and dehydroxylation (b) of all samples; the calculated activation energies are summarized in Tab. 3. The dehydration of CQIK took place at a somewhat higher temperature than that of the other clays. The increasing content of illite caused lower Tm of dehydroxylation. There was not any clear trend between the activation energy of individual processes in dependence on the clay composition.
The DSC curves of raw clays (Fig. 2) indicated other important information—they enable to relatively compare the energy needed for thermal activation of individual clays (Tab. 4). The first columns in Tab. 4 correspond to specific heat acquired from DSC data presented in Fig. 2, i.e. per g of the fresh sample. The values in the second column are related to g of clay minerals or CaCO3 in the fresh sample. Apparently, the dehydroxylation of kaolinite was more energy intensive than the dehydroxylation of illite and, obviously, the presence of inert minerals in the clay (quartz, calcite till 650 °C) reduced the energy needed for the thermal activation of the entire mixed clay.
Pozzolanic activity of activated mixed clays
The evaluation of the effectivity of thermal activation of mixed clays was performed by two methods: by the saturated lime test (performed for 168 h) and by measurement of strengths of cement pastes where 20% of OPC was replaced by the activated clay. The results of compressive strength (Fig. 8) indicate differences in the behaviour of individual activated clays. Generally, the best performance was observed in case of the sample K with the highest content of kaolinite. For this material, the best calcination temperature was determined to be 550 °C, but all of the pastes provided satisfactory results—the higher compressive strength than the control value (pure OPC paste with w/c 0.3). The clays IK, QIK and CQIK were more sensitive to the activation temperature—the outstanding performance was observed for 600 °C for IK and QIK, while the CQIK reached the best result for 650 °C. The bending strength (Fig. 9) was more sensitive to the calcination temperature than the compressive one, but the best values were observed for the same temperatures. The results of the Saturated Lime Test were less clear; the percentage of fixed Ca2+ reached for all samples K and IK in fact the same value around 95%. The results for QIK and CQIK were more distributed, but just in the case of sample CQIK the results of mechanical properties and SLT were in fair agreement.
Discussion
The series of mixed clays with different contents of kaolinite and illite (and quartz and calcite) were thermally activated within this work in order to obtain pozzolanic material. The thermal decomposition of clay minerals can be described by a sequence of steps [26]: Dehydration-dehydroxylation-amorphization-crystallization of new minerals. A clay mineral becomes a pozzolana when it undergoes dehydroxylation and consequent collapse of the layer structure to an amorphous matter; but—the formation of new minerals or glass has to be avoided. It means each clay has an optimum activation temperature, which depends (not only) on the content/proportion of present individual clay minerals. It is generally proven that kaolinite has the highest pozzolanic activity among clay minerals [7, 27, 28]. Skibsted and Snellings [26] explain this fact by the formation of a metastable phase—metakolinite—in kaolinitic clays; the metakolinite is stable over a wide range of temperature (ca. 450–850 °C). However, the optimum calcination temperature published in literature for kaolinitic clays ranges from 600 to 850 °C; obviously, the time of calcination and batch are also important variables [29]. Contrary, in 2:1 clay minerals any similar metaphase is not formed. This explanation has been confirmed also by the present results; the mixed clay K, with the highest kaolinite content, provided the most stable results, not significantly dependent on the activation temperature (Fig. 8). As the kaolinite content decreased—and illite increased—the “optimum” calcination temperature has become more pronounced, since illite (as 2:1 clay mineral) is more sensitive to a proper calcination temperature. This sensitivity is caused by the mentioned non-existence of a stable metaphase, but the structure of these clay minerals is continuously changing during the dehydroxylation [30]. It means that the highest reactivity is reached in just a narrow temperature range. The continuous breakdown of the illite structure was observable also by XRD (Figs. 5, 6) where residuals of the 002 diffraction line were observable even after calcination at 1000 °C when several new phases were formed in the system. The complete collapse of the illite structure was reported to take place at 950 °C [31]. The higher robustness of the kaolinite activation process is its huge advantage for its industrial production in rotary kilns when compared to other clay minerals since the control and homogeneity of batch temperature in a rotary kiln is more challenging than in a small electric laboratory furnace. The solution of this problem may be the proposed flash calcination [6].
The calcination at 600 °C seems to be optimal for samples with higher illite content IK, QIK and 650 °C for CQIK (Fig. 8), despite the structure of illite is not completely destroyed at this temperature. In terms of compressive strength, these three clays provided very similar results despite their different composition; they contain different amounts of nonreactive species (quartz, calcite): 26, 45 and 51%, respectively. The “optimum compressive strengths” of IK, QIK, CQIK calcined clays were somewhat lower than the “most-kaolinitic” K (81 vs. 88 MPa) but still well above the control value (pure OPC paste). The optimally calcined clays have improved more significantly the bending strength which implies that all the activated mixed clays were mitigating the shrinkage and cracking of the pastes; in general, the effect of calcined clays and other SCM on the shrinkage behaviour is not unambiguous [32].
The clay calcination temperature is obviously not important only with respect to the clay reactivity but also because of the energy consumption (and relevant emissions). The clay, supposed to be used as cement component, must be calcined separately at a proper—lower—temperature than the Portland clinker calcination takes place. The theoretical decomposition heat, as well as CO2 emissions, for 1:1, 2:1 and Portland clinker was published in [6]. From these published results, follow that 2:1 clays need less energy for the activation (529 vs. 1145 kJ kg−1). The clinker synthesis energy consumption is higher due to the higher calcination temperature (1780 kJ kg−1). By now, the clay calcination in a rotary kiln using a fossil fuel is likely the most viable way for industrial implementation of this technology. Considering the relevant CO2 emissions, the calcined clays benefits—compared to clinker—not only from the lower energy consumption but also from the absence (or a lower content) of carbonates in the raw material (whose calcination evolves further portion of CO2). Martinez et al. [33] performed environmental assessment of LC3 cement (50% Portland clinker, 15% limestone, 35% metakaolin) and concluded that this cement produced may potentially reduce greenhouse gases emissions by 5–50% compared to currently industrially produced cements (ordinary Portland cement, nowadays composite cements).
The comparison of the results obtained within this work and those published in the literature dealing with similar topics is provided in Tab. 5. Without any doubt, kaolinite is the best clay mineral in terms of Portland cement replacement. Nevertheless, the results of this work and e.g. [12, 31, 34, 36] show that also 2:1 clay minerals can be used successfully as SCM. There are also published results which are not such positive (mixed clays in [28, 35]), but it likely just indicates the above-mentioned sensitivity of 2:1 clay minerals to the proper activation temperature. The optimum calcination temperature for mixed clays found in the present research (600 °C and 650 °C, respectively) is well below the complete illite crystal structure destruction. However, the achieved strength results demonstrated that it is not necessary to reach the complete amorphization of the clay minerals. Obviously, the lower calcination temperature is favourable for energy consumption and also with respect to the unwanted thermal decomposition of carbonate minerals which are frequently present in the mixed clay raw materials; that is the case of calcitic brick soil CQIK calcined at 650 °C, i.e. bellow the massive carbonates decomposition. The content of “non-reactive impurities” in clay—namely quartz and calcite—is again not causing the reduction of ability to act as successful Portland cement replacement. This study, including the selection of clays, is very similar to reference [28] where higher (700 °C) activation temperature was used but authors of [28] dosed the calcined clays as 30% replacement thus it is not possible to clearly conclude.
The results of the saturated lime test (Fig. 8, Fig. 9) were not really reflecting the pozzolana performance in the cementitious binder. This method is based on the reaction between Ca2+ and OH− (i.e. dissolved Ca(OH)2) with a pozzolana to C–S–H and other hydration products. Consequently, the content of these ions in the solution is decreasing. Nevertheless, clay minerals are well-known as materials with high ionic exchange capacity. It means that Ca2+ ions from the solution may be adsorbed also by clay in its natural, inactivated state and thus saturated lime test (and other methods based on monitoring of Ca2+ content in solution) is not really reflecting the content of pozzolanic active species, but just the content of particles able to adsorb calcium ions. The amount of Ca2+ adsorbed by untreated illitic clay at pH 12.5 and 40 °C (i.e. conditions of saturated lime test) was 14 g.kg−1 [37]. It is a smaller amount than it is obtained as results of SLT (e.g. 95% of fixed Ca2+ corresponds to 76 g.kg−1) but still, this sorption capacity of raw clay may distort the results of SLT.
Conclusions
Four types of mixed illitic-kaolinitic clays with varied content of clay minerals, quartz and calcite were thermally activated in order to be used as supplementary cementing material. The main findings may be summarized as follows:
-
The optimum activation temperature for clay with the highest content of kaolinite (76%) was 550 °C, while the more illitic clays were successfully activated at 600 or 650 °C.
-
The crystal structure of illite was not completely broken down at 600 °C but still the ability of clay to replace Portland cement was very good. The lower calcination temperature is favourable with respect to energy consumption and potential unwanted decomposition of carbonate minerals present in the clay.
-
The performance of the calcined clay with the highest content of kaolinite was less sensitive to the activation temperature compared to illitic clays. This is related to the existence of a dehydroxylated metastable phase in kaolinitic clays (metakoalinite) while no such metastable phase is formed during the illite dehydroxylation. The illite dehydroxylation is a gradual process with a narrow range of the highest reactivity, prior to the new phases starting to crystallize.
-
The saturated lime test is not suitable for the evaluation of the pozzolanic activity of clay-based SCM since the Ca2+ cations are adsorbed also on the crystalline clay minerals.
References
Schneider M. Process technology for efficient and sustainable cement production. Cem Conc Res. 2015. https://doi.org/10.1016/j.cemconres.2015.05.014.
Gartner E, Sui T. Alternative cement clinkers. Cem Conc Res. 2018. https://doi.org/10.1016/j.cemconres.2017.02.002.
Almutairi AL, Tayeh BA, Adesina A, Isleem HF, Zeyad AM. Potential applications of geopolymer concrete in construction: a review. Case Stud Con Mat. 2021. https://doi.org/10.1016/j.cscm.2021.e00733.
Pacewska B, Wilinska I. Usage of supplementary cementitious materials: advantages and limitations. Part I. C–S–H, C–A–S–H and other products formed in different binding mixture. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09907-1.
Scrivener K, Martirena F, Bishnoi S, Maity S. Calcined clay limestone cements (LC3). Cem Conc Res. 2018. https://doi.org/10.1016/j.cemconres.2017.08.017.
Hanein T, Thienel K-C, Zunino F, Marsh ATM, Maier M, Wang B, Canut M, Juenger MCG, Ben Haha M, Avet F, Parashar A, Al-Jaberi LA, Almenares-Reyes RS, Alujas-Diaz A, Scrivener KL, Bernal SA, Provis JL, Sui T, Bishnoi S, Martirena-Hernández F. Clay calcination technology: state-of-the-art review by the RILEM TC 282-CCL. Mat Struct. 2022. https://doi.org/10.1617/s11527-021-01807-6.
Hollanders S, Adriaens R, Skibsted J, Cizer O, Elsen J. Pozzolanic reactivity of pure calcined clays. App Clay Sci. 2016. https://doi.org/10.1016/j.clay.2016.08.003.
Schulze SE, Rickert J. Suitability of natural calcined clays as supplementary cementitious material. Cem Conc Comp. 2019. https://doi.org/10.1016/j.cemconcomp.2018.07.006.
Rakhimova NR, Rakhimov RZ, Bikmukhametov AR, Morozov VP, Eskin AA, Lygina TZ, Gubaidullina AM. Role of clay minerals content and calcite in alkali activation of low-grade multimineral clays. J Mat Civ Eng. 2020. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003255.
Ruiz AI, Ruiz-García C, Ruiz-Hitzky E. From old to new inorganic materials for advanced applications: the paradigmatic example of the sepiolite clay mineral. App Clay Sci. 2023. https://doi.org/10.1016/j.clay.2023.106874.
Gou M, Zhou L, Then NWY. Utilization of tailings in cement and concrete: a review. Sci Eng Compos Mater. 2019. https://doi.org/10.1515/secm-2019-0029.
Bouchikhi A, Safhi AE, Rivad P, Snellings R, Abriak NE. Fluvial Sediments as SCMs: characterization, pozzolanic performance, and optimization of equivalent binder. J Mat Civ Eng. 2022. https://doi.org/10.1061/(ASCE)MT.1943-5533.0004071.
Zhang YL, Ling TC. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—a review. Con Build Mat. 2020. https://doi.org/10.1016/j.conbuildmat.2019.117424.
Diaz AD, Almenares Reyes RS, Hanein T, Irassar EF, Juenger M, Kanavaris F, Maier M, Marsh AT, Sui T, Thienel K-C, Valentini L, Wang B, Zunino F, Snellings R. Properties and occurrence of clay resources for use as supplementary cementitious materials: a paper of RILEM TC 282-CCL. Mat Struct. 2022. https://doi.org/10.1617/s11527-022-01972-2.
Arab PB, Araújo TP, Pejon OJ. Identification of clay minerals in mixtures subjected to differential thermal and thermogravimetry analyses and methylene blue adsorption tests. App Clay Sci. 2015. https://doi.org/10.1016/j.clay.2015.05.020.
Ondro T, Al-Shantir O, Csaki S, Lukac F, Trnik A. Kinetic analysis of sinter-crystallization of mullite and cristobalite from kaolinite. Thermochim Acta. 2019. https://doi.org/10.1016/j.tca.2019.178312.
Malata G, Tkaczewska E. Application of thermal methods in the studies of potential pozzolanic reactivity of attapulgite and sepiolite. J Therm Anal Calorim. 2023. https://doi.org/10.1007/s10973-023-12257-3.
Döbelin N, Kleeberg R. Profex: a graphical user interface for the Rietveld refinement program BGMN. J App Crystallography. 2015. https://doi.org/10.1107/S1600576715014685.
Donatello S, Tyrer M, Cheeseman CR. Comparison of test methods to assess pozzolanic activity. Cem Conc Comp. 2010. https://doi.org/10.1016/j.cemconcomp.2009.10.008.
Ptáček P, Kubátová D, Havlica J, Brandštetr J, Šoukal F, OPravil T. The non-isothermal kinetic analysis of the thermal decomposition of kaolinite by thermogravimetric analysis. Powder Tech. 2010. https://doi.org/10.1016/j.powtec.2010.08.004.
Ondruška J, Csáki Š, Štubňa I, Trnovcová V. Investigation of kaolin–quartz mixtures during heating using thermodilatometry and DC thermoconductometry. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-019-08476-2.
Földvári M. Handbook of thermogravimetric system of minerals and its use in geological practice. Budapest: Geological Institute of Hungary; 2011.
Vimmrová A, Krejsová J, Scheinherrová L, Doleželová M, Keppert M. Changes in structure and composition of gypsum paste at elevated temperatures. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09528-8.
Derkowski A, Kuligiewicz A. Thermal analysis and thermal reactions of smectites: a review of methodology, mechanisms, and kinetics. Clays Clay Miner. 2022. https://doi.org/10.1007/s42860-023-00222-y.
Šimon P, Dubaj T, Cibulková Z. Frequent flaws encountered in the manuscripts of kinetic papers. J Therm Anal Calorim. 2022. https://doi.org/10.1007/s10973-022-11436-y.
Skibsted J, Snellings R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem Conc Res. 2019. https://doi.org/10.1016/j.cemconres.2019.105799.
Fernandez R, Martinera F, Scrivener KL. The origin of the pozzolanic activity of calcined clay minerals: a comparison between kaolinite, illite and montmorillonite. Cem Conc Res. 2011. https://doi.org/10.1016/j.cemconres.2010.09.013.
Tironi A, Trezza MA, Scian AN, Irassar EF. Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Con Build Mat. 2012. https://doi.org/10.1016/j.conbuildmat.2011.08.064.
Rashad AM. Metakaolin as cementitious material: History, scours, production and composition—a comprehensive overview. Con Build Mat. 2013. https://doi.org/10.1016/j.conbuildmat.2012.12.001.
Garg N, Skibsted J. Thermal activation of a pure montmorillonite clay and its reactivity in cementitious systems. J Phys Chem C. 2014. https://doi.org/10.1021/jp502529d.
Irassar EF, Bonavetti VL, Castellano CC, Trezza MA, Rahhal VF, Cordoba G, Lemma R. Calcined illite-chlorite shale as supplementary cementing material: thermal treatment, grinding, color and pozzolanic activity. App Clay Sci. 2019. https://doi.org/10.1016/j.clay.2019.105143.
Weng J-R, Liao W-C. Microstructure and shrinkage behavior of high-performance concrete containing supplementary cementitious materials. Con Build Mat. 2021. https://doi.org/10.1016/j.conbuildmat.2021.125045.
Martinez DM, Horvath A, Monteiro PJM. Comparative environmental assessment of limestone calcined clay cements and typical blended cements. Env Res Comm. 2023. https://doi.org/10.1088/2515-7620/acccd8.
Mohammed S, Ghorbel E, Mekki B. Valorization of pozzolanicity of Algerian clay: optimization of the heat treatment and mechanical characteristics of the involved cement mortars. App Clay Sci. 2016. https://doi.org/10.1016/j.clay.2016.08.027.
Chikouche MA, Ghorbel E, Mekki B. The possibility of using dredging sludge in manufacturing cements: optimization of heat treatment cycle and ratio replacement. Con Build Mat. 2016. https://doi.org/10.1016/j.conbuildmat.2015.12.128.
Poussardin V, Paris M, Wilson W, Tagnit-Hamou A, Deneele D. Calcined palygorskite and smectite bearing marlstones as supplementary cementitious materials. Mat Struct. 2022. https://doi.org/10.1617/s11527-022-02053-0.
Cherian C, Kollannur NJ, Bandipally S, Arnepalli DN. Calcium adsorption on clays: effects of mineralogy, pore fluid chemistry and temperature. App Clay Sci. 2018. https://doi.org/10.1016/j.clay.2018.02.034.
Acknowledgements
This research has been supported by the Czech Science Foundation, under project No 22-16577S and by the Czech Technical University under project SGS22/137/OHK1/3T/11.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
Martin Keppert prepared writing—original draft. Vojtěch Pommer did investigation. Kateřina Šádková conducted investigation. Jitka Krejsová presented data curation. Eva Vejmelková performed data curation. Robert Černý provided resources. Dana Koňáková revised writing—review & editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keppert, M., Pommer, V., Šádková, K. et al. Thermal activation of illitic-kaolinitic mixed clays. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13342-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13342-x