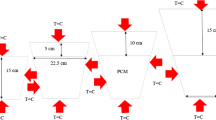
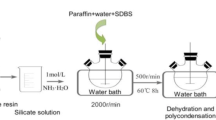
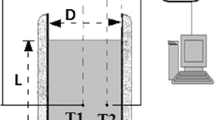
Abstract
Phase change materials (PCMs) are capable of thermal energy storage since they have a set melting point and a high latent heat of melting. PCMs offer up to 15 times the heat capacity per unit volume compared to conventional storage materials. The results show that laboratory methods were expensive and time-consuming. Therefore, using the molecular dynamics (MD) simulation method, this study investigated the effect of external heat flux (EHF) (0.001–0.005 W m−2) and structural type (paraffin (S2) and sodium sulfate/magnesium chloride hexahydrate (S1)) on the thermal behavior (TB) and phase change process (PCP). The effect of these two factors was investigated on the temperature profile, heat flux (HF), charge, and discharge times. The results showed that the thermal and atomic properties of S1 are better than S2. As a result, the composite of two PCMs improves the properties. Due to its performance and safety, it can be used in industries. On the other hand, the EHF in S1 increases the HF, temperature, charge, and discharge time.











Similar content being viewed by others
Abbreviations
- PCM:
-
Phase change material
- S1:
-
Sodium sulfate/magnesium chloride hexahydrate
- S2:
-
Paraffin
- TB:
-
Thermal behavior
- PCP:
-
Phase change process
- HF:
-
Heat flux
- MD:
-
Molecular dynamics
- EHF:
-
External heat flux
- a i :
-
Acceleration of the particle (m s−2)
- ρ β :
-
An attraction force caused by the presence of particles in the simulated box
- ϕ β :
-
A repulsive force caused by atomic charge density.
- k B :
-
Boltzmann constant (1.380649 × 10−23 J K−1)
- F α :
-
Constant coefficient between 0 and 1
- ε ij :
-
Depth of the potential well (kJ mol−1)
- r ij :
-
Distance between particles (m)
- r :
-
Distance of the particles from each other
- U ij :
-
Electric potential (eV)
- σ ij :
-
Finite distance in which the potential is zero (Å)
- J :
-
Heat flux (Wm−2)
- m i :
-
Mass of the particle (g)
- N fs :
-
Number of degrees of freedom
- u i :
-
Potential of a particle (eV)
- T :
-
System temperature (K)
- V :
-
Total volume of particles (Å3)
References
Ismail KA, Lino FA, Machado PLO, Teggar M, Arıcı M, Alves TA, Teles MP. New potential applications of phase change materials: a review. J Energy Storage. 2022. https://doi.org/10.1016/j.est.2022.105202.
Sikiru S, Oladosu TL, Amosa TI, Kolawole SY, Soleimani H. Recent advances and impact of phase change materials on solar energy: a comprehensive review. J Energy Storage. 2022. https://doi.org/10.1016/j.est.2022.105200.
Zhou T, Xiao Y, Liu Y, Lin J, Huang H. Research on cooling performance of phase change material-filled earth-air heat exchanger. Energy Convers Manag. 2018. https://doi.org/10.1016/j.enconman.2018.09.047.
He Q, Wang S, Tong M, Liu Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers Manag. 2012. https://doi.org/10.1016/j.enconman.2012.04.010.
Jebasingh BE, Arasu AV. A detailed review on heat transfer rate, supercooling, thermal stability and reliability of nanoparticle dispersed organic phase change material for low-temperature applications. Mater Today Energy. 2020. https://doi.org/10.1016/j.mtener.2020.100408.
Umair MM, Zhang Y, Iqbal K, Zhang S, Tang B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—a review. Appl Energy. 2019. https://doi.org/10.1016/j.apenergy.2018.11.017.
Li T, Wu D, He F, Wang R. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage. Int J Heat Mass Transf. 2017. https://doi.org/10.1016/j.ijheatmasstransfer.2017.07.056.
Zhang C, Zhang Z, Ye R, Gao X, Ling Z. Characterization of MgCl2· 6H2O-based eutectic/expanded perlite composite phase change material with low thermal conductivity. Mater. 2018. https://doi.org/10.3390/ma11122369.
Kazemi A, Naseri I, Nasiri M, Bahramian AR. Effect of MgCl2· 6H2O phase change material on thermal insulation performance of carbon aerogels. J Energy Storage. 2017. https://doi.org/10.1016/j.est.2016.12.002.
Dong Y, Wang F, Zhang Y, Shi X, Zhang A, Shuai Y. Experimental and numerical study on flow characteristic and thermal performance of macro-capsules phase change material with biomimetic oval structure. Energy. 2022. https://doi.org/10.1016/j.energy.2021.121830.
Fan Z, Gao Q, Zhao T, Wu Y, Zhang C, Li Z. Preparation and properties of a novel composite phase change material with low supercooling and high thermal conductivity. Soc Sci Res Netw (SSRN). 2023. https://doi.org/10.2139/ssrn.4328138.
Wang J, Yin Y, Wang Y, Huang J. Thermal performance analysis of multi-stage cold storage packed bed with modified phase change material based on Na2SO4· 10H2O. Appl Therm Eng. 2023. https://doi.org/10.1016/j.applthermaleng.2022.119666.
Kalidasan B, Pandey A, Saidur R, Samykano M, Tyagi V. Nano additive enhanced salt hydrate phase change materials for thermal energy storage. Int Mater Rev. 2022. https://doi.org/10.1080/09506608.2022.2053774.
Bhoi S, Banerjee T, Mohanty K. Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF. Fuel. 2014. https://doi.org/10.1016/j.fuel.2014.07.058.
Qiu Y, Zhong W, Shao Y, Yu A. Reactive force field molecular dynamics (ReaxFF MD) simulation of coal oxy-fuel combustion. Powder Technol. 2020. https://doi.org/10.1016/j.powtec.2019.07.103.
Yu Y, Tao Y, He Y-L. Molecular dynamics simulation of thermophysical properties of NaCl–SiO2 based molten salt composite phase change materials. Appl Therm Eng. 2020. https://doi.org/10.1016/j.applthermaleng.2019.114628.
Li Q, Yu Y, Liu Y, Liu C, Lin L. Thermal properties of the mixed n-octadecane/Cu nanoparticle nanofluids during phase transition: a molecular dynamics study. Materials. 2017. https://doi.org/10.3390/ma10010038.
Liu X, Adibi M, Shahgholi M, Tlili I, Sajadi SM, Abdollahi A, Li Z, Karimipour A. Phase change process in a porous carbon–paraffin matrix with different volume fractions of copper oxide nanoparticles: a molecular dynamics study. J Mol Liq. 2022. https://doi.org/10.1016/j.molliq.2022.120296.
Du Y, Zhou T, Zhao C, Ding Y. Molecular dynamics simulation on thermal enhancement for carbon nano tubes (CNTs) based phase change materials (PCMs). Int J Heat Mass Transf. 2022. https://doi.org/10.1016/j.ijheatmasstransfer.2021.122017.
Zhao C, Tao Y, Yu Y. Thermal conductivity enhancement of phase change material with charged nanoparticle: a molecular dynamics simulation. Energy. 2022. https://doi.org/10.1016/j.energy.2021.123033.
Rao Z, Wang S, Peng F. Molecular dynamics simulations of nano-encapsulated and nanoparticle-enhanced thermal energy storage phase change materials. Int J Heat Mass Transf. 2013. https://doi.org/10.1016/j.ijheatmasstransfer.2013.07.065.
Yan X, Zhao H, Feng Y, Qiu L, Lin L, Zhang X, Ohara T. Excellent heat transfer and phase transformation performance of erythritol/graphene composite phase change materials. Compos B Eng. 2022. https://doi.org/10.1016/j.compositesb.2021.109435.
Zhang S, Jin Y, Yan Y. Depression of melting point and latent heat of molten salts as inorganic phase change material: Size effect and mechanism. J Mol Liq. 2022. https://doi.org/10.1016/j.molliq.2021.117058.
Rapaport DC, Rapaport DCR. The art of molecular dynamics simulation. Cambridge: Cambridge University Press; 2004.
Swope WC, Andersen HC, Berens PH, Wilson KR. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters. J Chem Phys. 1982. https://doi.org/10.1063/1.442716.
Hairer E, Lubich C, Wanner G. Geometric numerical integration illustrated by the Störmer–Verlet method. Acta Numer. 2003. https://doi.org/10.1017/S0962492902000144.
Dunikov D, Malyshenko S, Zhakhovskii V. Corresponding states law and molecular dynamics simulations of the Lennard–Jones fluid. J Chem Phys. 2001. https://doi.org/10.1063/1.1396674.
Berendsen H, Grigera J, Straatsma T. The missing term in effective pair potentials. J Chem Phys. 1987. https://doi.org/10.1021/j100308a038.
Rappé AK, Casewit CJ, Colwell K, Goddard WA III, Skiff WM. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc. 1992. https://doi.org/10.1021/ja00051a040.
Mosavi A, Hekmatifar M, Alizadeh AA, Toghraie D, Sabetvand R, Karimipour A. The molecular dynamics simulation of thermal manner of Ar/Cu nanofluid flow: the effects of spherical barriers size. J Mol Liq. 2020;319:114183. https://doi.org/10.1016/j.molliq.2020.114183.
Daw MS, Baskes MI. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys Rev B. 1984. https://doi.org/10.1103/PhysRevB.29.6443.
Cao J, Liu L, Liu C, He C. Phase transition mechanisms of paraffin in waxy crude oil in the absence and presence of pour point depressant. J Mol Liq. 2022. https://doi.org/10.1016/j.molliq.2021.116989.
Zhang YX, Alizadeh AA, Abed AM, Nasajpour-Esfahani N, Smaisim GF, Hadrawi SK, Zekri H, Baghaei S, Esmaeili S, Wang MX. Investigating the effect of size and number of layers of iron nanochannel on the thermal behavior and phase change process of calcium chloride/sodium sulfate hexa-hydrate with molecular dynamics simulation. J Energy Storage. 2023;62:106762. https://doi.org/10.1016/j.est.2023.106762.
Fethi A, Mohamed L, Mustapha K, Sassi BN. Investigation of a graphite/paraffin phase change composite. Int J Therm Sci. 2015. https://doi.org/10.1016/j.ijthermalsci.2014.09.008.
Pan D, Ovcharenko A, Song W, Qi X. Investigation of lubricant depletion under a continuous heat source using molecular dynamics simulation. Microsyst Technol. 2018. https://doi.org/10.1007/s00542-018-3842-1.
Regin AF, Solanki S, Saini J. Heat transfer characteristics of thermal energy storage system using PCM capsules: a review. Renew Sust Energ Rev. 2008. https://doi.org/10.1016/j.rser.2007.06.009.
Rathod MK, Banerjee J. Thermal stability of phase change materials used in latent heat energy storage systems: a review. Renew Sust Energ Rev. 2013. https://doi.org/10.1016/j.rser.2012.10.022.
Zalba B, Marın JM, Cabeza LF, Mehling H. Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl Therm Eng. 2003. https://doi.org/10.1016/S1359-4311(02)00192-8.
Sharma A, Tyagi VV, Chen CR, Buddhi D. Review on thermal energy storage with phase change materials and applications. Renew Sust Energ Rev. 2009. https://doi.org/10.1016/j.rser.2007.10.005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamani, M., Toghraie, D., Mehmandoust, B. et al. Investigating the effect of external heat flux and atomic structure on the thermal behavior and phase change process of sodium sulfate/magnesium chloride hexahydrate and paraffin with molecular dynamics simulation. J Therm Anal Calorim 149, 2199–2207 (2024). https://doi.org/10.1007/s10973-023-12826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12826-6