Abstract
Introducing supplementary cementitious materials (SCM), e.g. fly ash, into cement composite results in ecological benefits. However, in the case of high amount of SCM used as a replacement of a part of cement, there are problems related to the development of the desired properties of the final composite. Such mixtures often require activation. In the first part of this series of publications, the results of chemical activation (using Na2SO4 and Ca(OH)2) of a mixture with a very high content of fly ash were discussed. The aim of this work was to investigate the influence of mechanical activation on hydration and microstructure of the binder composed of 80% of fly ash and 20% of cement. Mechanical activation was performed using a planetary ball mill. The following instrumental methods were used to investigate the activated fly ash-cement pastes: calorimetry, TG/DTG, FTIR spectroscopy, X-ray diffraction and scanning electron microscopy (SEM) coupled with EDS. It was shown that concomitant grinding of cement and fly ash is more effective compared to separate grinding. Mechanism of hydration/activation of such mixtures was discussed in detail.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Portland cement, which was invented in the nineteenth century, is still a very important binding construction material today. After mixing with proper amount of water, fine and coarse aggregate, it creates an initially workable mixture, and then hardened concrete as a result of physicochemical hydration processes [1]. However, it is well known that the production of Portland cement is not environmentally friendly as it consumes a lot of energy, uses natural resources and emits large amounts of CO2 [1,2,3,4]. Green concrete is a type of concrete designed to reduce the negative impact of cement production on the environment by the use of alternative materials or recycled agricultural, industrial or municipal waste materials. In this way, such kind of concrete contributes to reduction of CO2 emission, conservation of natural resources and also decreasing the area of landfills. However, despite numerous advantages of green concrete, there are also major barriers limiting its utilization in construction, such as: the need to obtain a sufficiently high strength and durability of the final material, low quality of available materials, standard limitations, technical problems and insufficient data relating to long-term durability. Moreover, waste materials used in the production of green concrete often require activation to increase their pozzolanic or hydraulic activity [5].
One of the possibilities to obtain green concrete is using supplementary cementitious materials (SCM), among which fly ash is the most common, to replace a part of cement in the concrete. Small and moderate amounts of fly ash can advantageously modify the properties of the cement composite, but the use of very high volumes of fly ash (VHVFA), i.e. 70–80%, significantly worsens them. In such situation, activation of the system may be necessary to obtain satisfactory properties of the final material.
There are different ways to activate fly ash and other pozzolanic materials. These methods can be divided into: chemical, mechanical and thermal activation. A combination of them is also possible and can result with an enhancement of the activation effect. Various ways of activation of mixtures containing high and very high amount of fly ash were discussed in our review paper [6].
Mechanical activation consists in supplying the system with mechanical energy (as an effect of grinding), resulting in physicochemical transformations of the activated substance. During the mechanical activation, the grains of the material are being broken down, they become smaller and of higher specific surface area, altered shape and morphology [7,8,9].
Mechanical activation can result in improvement of bulk and surface reactivity of the material. Depending on the conditions of activation process, the overall chemistry of the solid material may not be altered [10] or some changes in the structure, chemical and phase composition can take place leading to metastable product [7,8,9,10,11]. Thus, in the case of fly ash, there are three options of mechanical activation of its grains [12]: 1) mechanical dispersion, reduction in the grains diameter and increase in their specific surface area, 2) activation of the surface of the grains, 3) changes in chemical structure of internal components of fly ash particles. The amorphization of the activated material, especially during long-term milling, was observed by the authors of [9,10,11,12,13]. On the other hand, some authors reported that mechanical activation of fly ash leads to reduction in amorphous phase [14].
It is clear that the effectiveness of activation, including the degree of fragmentation, depends on the conditions of the process, such as the type of milled equipment, the size of the grinding balls, the method of grinding (dry or wet), rotational speed and time of milling. In general, the grinding process results in gradual transformation of spherical grains of fly ash in smaller particles of heterogeneous shape. Increasing time of the activation is mainly effective in reducing the diameter of bigger size grains [14]. Overall, the grain size is initially significantly reduced even during a relatively short time of mechanical activation. A longer grinding time results in a further reduction in particle size, although to a lesser extent, compared to the effect of the shorter grinding time, whereas extended grinding (e.g. 4 h in [15], 24 h used in [9]) causes no further reduction in grain size and may even result in its increase. This is the effect of creation of aggregates by very fine fly ash grains [9, 15, 16]. Du et al. indicated in their review article [17] that optimum grinding time amounts about 2 h, while longer duration of grinding does not further reduce the size of fly ash grains. Marjanović et al. [16] affirmed that 15 min. of mechanical activation is sufficient for prominent changes in granulometric composition of fly ash grains. A few authors indicated structural rearrangement and amorphization of fly ash grains as well as transformation towards nanostructured material during long-term mechanical activation (from a dozen or so to several dozen hours) in high-energy planetary ball mill [7, 13, 18, 19].
Mechanical activation can be realized in two ways: as separate grinding (the fly ash is grinded individually and then it is mixed with cement) or intergrinding (both the components, i.e. fly ash and cement, are milled concomitantly in the same grinding bowl). In the case of the latter kind of activation, the strong mechanical interaction between fly ash and cement particles takes place [20]. The occurring physical interactions mainly depend on relative difference in grindability of the milled constituents [21]. This is why the grains size, their shape and morphology can be different when comparing results of activation by separate grinding and intergrinding [20, 21]. Intergrinding process is considered more effective and it can be less energy-demanding compared to separate milling [21, 22].
In summary, it should be affirmed that mechanical activation is considered to be highly effective method and is often used to improve fly ash reactivity [23]. Activation by grinding results in acceleration of dissolution of fly ash active components in the hardening cement system, enhancement of pozzolanic reaction, improvement of precipitation of hydration products favouring development of more compact microstructure and, as a result of all of this, higher mechanical strength of the cement composites containing typical amount of fly ash [23, 24] and those containing HVFA [24, 25]. However, there is less work on the mechanical activation of VHVFA binders, and this process is not well understood yet. Understanding the grinding process and its influence on hydration and microstructure of activated binders is a necessary step to predict the properties of VHVFA composites and develop a new eco-binders showing required properties.
Mechanical activation has the following advantages: the process is simple and makes it possible to obtain products of modified properties [11], however, the effectiveness of this type of activation is limited in the case of VHVFA binders. This is because Class F fly ash needs alkaline component to develop its activity. In the case of VHVFA binders, Ca(OH)2 is provided by the hydration of cement. However, the amount of cement in such compositions is low, thus the amount of Ca(OH)2 is also limited. As a result, mechanical activation can improve reactivity of the fly ash and the properties of the final composite but only to a limited extent. Introduction of additional chemical activators is needed in the case when the properties of VHVFA mixture after mechanical activation are not satisfactory.
Previously, we investigated the hydration processes of chemically activated fly ash–cement mixtures containing VHVFA [6, 26, 27] as well as cement pastes containing typical amount of fly ash [28]. Preliminary calorimetric results of mechanical activation of fly ash–cement mixture by intergrinding for 10, 30 and 40 min. were previously presented in [6, 27]. Interesting results were obtained in the case of calorimetric measurements of VHVFA binders activated simultaneously in chemical and mechanical way. In this case, the chemical activation consisted of the introduction of small amount of Na2SO4 and Ca(OH)2 into the system, while mechanical activation consisted of joint grinding of all the dry components before the addition of water [6, 27]. This prompted us to investigate the hydration mechanism of this activated system in more detail. For this purpose, we tested the systems of fly ash + Portland cement + Ca(OH)2 + Na2SO4 not mechanically activated (results of which were described in the first part of this work cycle [26]), VHVFA mixtures only mechanically activated (the second part of the cycle—this work) and VHVFA chemically and mechanically activated systems by the use of concomitant grinding of fly ash, cement and chemical activators (the results will be discussed in the third part of the publication). The stage of the research, the results of which are presented in this paper, is necessary to understand the mechanism of action of such chemical–mechanical activation. The studies contribute to broadening the knowledge on the mechanism of mechanical activation of mixtures containing very high amount of fly ash. The results make for better understanding of the chemistry of green concrete and relation between the chemical transformations and properties of the final composite.
The aim of this study was to investigate the influence of the mechanical activation on hydration of the VHVFA binder and development of its microstructure. Results of such investigations for VHVFA mixtures are rarely presented in the literature especially when intergrinding is considered. In this paper, joint grinding of fly ash and cement is proposed as a method leading to greater activity of the system. In this way, both components of VHVFA mixture can be activated using planetary ball mill, surface properties of their grains can be altered and the synergy effect allows for better results. Various grinding times but not exceeding 60 min. were used to obtain ecologically friendly cementitious material. Moreover, to better understand the mechanical activation process, separate milling was also used for selected samples.
Materials and methods
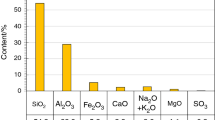
The following materials were used to prepare fly ash–cement pastes: (1) commercially available Portland cement CEM I 32,5R with properties in accordance with the standard PN-EN 197–1 and (2) Class F fly ash from conventional pulverized combustion of hard coal in energy industry. The chemical composition of fly ash was presented in the previous work [26]. Granulometric composition of fly ash can be found in [6]. The materials were weighed in the same mass ratio as in the previous work [26], i.e. 80% of fly ash and 20% of cement, and then mixed. Chemical composition of the mixture was as follows (mass %): SiO2 46.0, Al2O3 23.7, Fe2O3 4.9, SO3 0.9, CaO 15.5, Na2O + K2O 2.4, TiO2 1.16, MgO 1.3.
Fly ash and cement were mechanically activated prior to the addition of water. Planetary ball mill PM 100 (Retsch) was used. The milling was performed in a dry state and in an air atmosphere using grinding jar and 10 mm-size grinding balls made of zirconia. Rotation speed was 350 rpm. Two ways of activation were used, i.e. separate grinding and intergrinding. In the case of the first mentioned method of activation, dry materials were ground individually (separately) for 30 min., while in the case of the second way, the components in the mass proportion 80% of fly ash and 20% of cement were milled concomitantly for a different period of time from 5 up to 60 min. The grain size distribution of the fly ash–cement mixtures was tested with the use of laser particle size analyser. Shape and morphology of the grains were analysed using SEM microscope Merlin FE-SEM/EDS (Carl Zeiss). Thermal analysis, FITR spectroscopy and X-ray diffraction tests were performed using the same apparatus as in the case of study of fly ash–cement pastes, described below.
Fly ash–cement pastes were performed with distilled water (water to solid ratio was 0.5). Pastes intended for calorimetric tests were hydrated in calorimeter at 25 °C for 48 h. Pastes intended for other tests were poured into small polyethylene bags and sealed. Then they were stored at room temperature. After the appointed period of hydration, the pastes were removed from the bags, ground, and hydration was stopped with acetone.
The following notations of the samples were used:
in the case of separate grinding: 80FA(30)/20PC, 80FA/20PC(30) and 80FA(30)/20PC(30) for the mixture containing activated fly ash, activated cement or both activated components, respectively (the grinding time is given in parentheses); in the case of intergrinding: 80FA/20PC-x, where x means the time of grinding.
Research methods and apparatus that were used in this work to test fly ash–cement pastes:
-
calorimetric measurements—BMR calorimeter constructed at the Institute of Physical Chemistry, Polish Academy of Sciences, the results were calculated using computer software [29],
-
thermal analysis—TA 2500 Regulus (Netzsch) thermoanalyser, the temperature range of the tests: from 30 to 900 °C, a nitrogen atmosphere, the rate of heating: 10 °C min−1, the mass of the sample: 20 ± 3 mg,
-
infrared spectroscopy—FTIR Genesis II (Mattson) spectrometer, the range of wavenumbers: 4000–400 cm−1, KBr pellets,
-
X-ray diffraction—Bruker D8 Advance diffractometer, Cu-Kα radiation,
-
SEM/EDS analysis—scanning electron microscope JEOL with an X-ray microanalyzer EDS.
Results and discussion
Investigation of dry mixtures of fly ash and cement
To investigate the physical and chemical changes that occur during mechanical activation of fly ash–cement mixtures, several tests were performed: analysis of granulometric compositions of the samples, thermal analysis, X-ray diffraction patterns and FTIR spectra were collected.
The influence of the time of grinding on granulometric composition of fly ash–cement mixtures is depicted in Fig. 1. It is clearly visible that even short grinding time (10 min.) results in a significant reduction in the average and maximum diameter of fly ash grains. The degradation efficiency increases with the time of milling, however, comparing the results for 30 and 60 min. of grinding it is visible that the reduction in particle size during prolonged time of milling is not so intense as in the case of the first minutes of the process. This is because the smaller grains of fly ash are more difficult to degrade during grinding. Nevertheless, it is apparent that long grinding time (60 min.) increases the amount of very fine grains with a diameter between 0.7 and 5 μm and the maximum grain size does not exceed 60 μm in this case. The share of the fraction with a grain diameter below 45 μm is: 58, 84, 97 and 99% for the mixtures 80FA/20PC-0, 80FA/20PC-10, 80FA/20PC-30, 80FA/20PC-60, respectively. Such particle size is preferred when fly ash is used as supplementary cementitious material, because particles of diameter below 45 μm tend to increase mechanical strength of the final composite [15, 16].
SEM observations made it possible to evaluate the shape of particles of the mixtures before and after grinding. The results are presented in Fig. 2. It is visible that, especially for the reference mixture, the particles vary both in shapes and sizes. This binder contains mainly spherical fly ash grains of different diameter from over 100 μm to below 1 μm. Irregular grains of cement, existing in the mixtures in smaller amount compared to fly ash, are also visible.
The SEM images show that the mechanically activated specimens are composed of smaller and more irregular grains compared to the reference mixture. The results confirm that larger grains are mainly susceptible to grinding process, while small spherical grains remain unchanged. This is why, the sample milled for 60 min. (80FA/20PC-60) is mainly composed of grains up to 30 μm in size—irregular and small spherical particles are visible. This sample shows the greatest comminution, which results from the longest grinding time.
The results of thermal analysis, X-ray diffraction and FTIR spectroscopy showed that the milling process in conditions adopted in this work does not affect the phase composition of the samples significantly. Thus, the results were presented only for the fly ash–cement mixtures activated during short (10 min.) and long (60 min.) time compared to the reference mixture.
Thermal analysis results (Fig. 3) registered for fly ash–cement mixtures show that the materials are thermally stable, as evidenced by a low total mass loss: 1.35% for unmilled sample, 1.09 and 1.5% for mixtures ground for 10 and 60 min., respectively. The total mass loss can be divided into three main periods: (1) till 370 °C (evaporation of humidity, dehydration of gypsum and hydrated silicate phases with could be formed during storage), (2) 370–450 °C (dehydroxylation of Ca(OH)2 which also could have been created in a result of storage and contact with air humidity), (3) above 550 °C till about 730 °C (decomposition of carbonates). The mass loss observed above 800 °C for TG/DTG curves of the ground specimens may indicate reduction of CaSO4 and/or Fe2O3 with carbon [30,31,32].
Grinding process promotes evaporation of water from the sample and the formation of carbonates in reaction with CO2. The amount of Ca(OH)2 is almost the same in all the mixtures.
XRD patterns for fly ash–cement mixtures are depicted in Fig. 4. The presence of the components coming from fly ash (quartz, mullite) and from cement (alite) are visible. Other components of cement occur in smaller amounts than alite, thus their presence was not registered in the X-ray patterns. The kinds of the crystalline components are the same in all mixtures and the intensities of the registered effects also do not differ significantly from sample to sample. Thus, the results show that mechanical activation did not change the composition of the fly ash–cement mixture in the range of crystalline phases.
IR spectroscopy, which results are presented in Fig. 5, can supplement the results of X-ray diffraction because the presence of not only crystalline phases can be visible in this way. As could be expected, the absorption bands observed in the IR spectra result mainly from the presence of fly ash in the samples, and they overlap with the smaller intensity bands typical for cement. Intense band with an extreme slightly below 1100 cm−1 is the most visible. It relates to asymmetric stretching vibrations of Si(Al)–O bonds [33,34,35] in silica and aluminosilicate phases included in fly ash (cumulative signals of quartz, mullite, amorphous phase). This broad band overlaps the bands coming from presence of cement and makes them invisible (e.g. alite at 935 cm−1, calcium sulphate at about 1100 cm−1 [36]). The shoulder at about 1160 cm−1 indicates the presence of silica (quartz) [16, 34]. The weak intensity band at 797 cm−1 can also be attributed to Si–O vibrations in quartz [16, 34], stretching vibrations of AlO4 and vibrations of amorphous aluminosilicate phase showing ring structures as well [37, 38]. Other bands due to the presence of quartz in the sample are located at 668, 690 and about 460 cm−1 while the effect at 545 cm−1 may be due to overlapping bands of the vibrations in quartz and mullite molecules [34]. The broad medium intensity band at the range of wavenumbers from 3200 to 3700 cm−1 relates to stretching vibrations of H–O–H while small intensity band at about 1635 cm−1 results from bending vibrations of water molecules. The presence of carbonates is confirmed by the bands at 1420 and 876 cm−1.
The shapes of the spectra presented in Fig. 5 are very similar which indicate that the compositions of samples are not altered significantly during grinding process. However, one can see that in the case of 80FA/20PC-60 the main fly ash band is slightly broadened and its extreme shifts to higher wavenumbers compared to the spectra registered for other mixtures. Moreover, the bands at 797 and 668 cm−1 are less visible in the case of 80FA/20PC-60 sample. The changes show some rearrangements in aluminosilicate structure of the mixture milled for 60 min. and some increase in the degree of amorphization of the sample.
Similar to the XRD results, IR spectra did not reveal the formation of new phases during mechanical activation of the samples. Only small increase in degree of amorphization of the sample can take place in the case of long milling time. Thus the changes in fly ash–cement activity in the presence of water result mainly from the reduction in the size of the grains, damaging their outer layers and thus allowing the ions to pass into solution more easily.
Investigation of VHVFA pastes
Calorimetric measurements
The results of calorimetric tests are presented in Figs. 6 and 7. The typical shape of the curve of heat release rate during early hydration of Portland cement is well-known and described in the literature, e.g. [39,40,41,42]. Five characteristic periods can be distinguished on this curve following one after the other: wetting period, induction period, acceleration and then deceleration periods, finally the stage of slow continued reactions. Exchange of a part of cement by fly ash usually results in elongation of the induction period and reduction in heat release rate and total heat released [6, 27]. This is because fly ash needs time to reveal its pozzolanic properties in cement paste. Thus, at the early periods after adding water to the fly ash–cement mixture, cement undergoes hydration processes while fly ash is initially unreactive [43]. Although fine grains of fly ash may accelerate cement hydration (nucleation effect) and ions washed out from the fly ash may affect the course of the process, the effects do not compensate for the reduction in cement amount in the system, especially when HVFA and VHVFA mixtures are considered.
However, it should be noted that the influence of fly ash on cement hydration kinetics is more complex, which is also due to the mechanical activation process. In general, fly ash affects the cement hydration in different chemical and physical ways. Some of the interactions accelerate the hydration processes, while other decelerate them. The final result depends on which phenomenon predominates.
Typical shape of calorimetric curve registered for non-activated fly ash–cement mixture (80FA/20PC-0) is presented in Fig. 6. Other curves depicted in this figure show the heat release rate for fly ash–cement mixtures after 30 min. of activation in different ways: the cement was mechanically activated while fly ash was used as received (80FA/20PC(30)), fly ash was milled while cement was used without activation (80FA(30)/20PC), both the components were activated individually and then mixed by hand before addition of water (80FA(30)/20PC(30)) and finally fly ash and cement were ground concomitantly (80FA/20PC-30). The results show that the way of mechanical activation influences the rate of heat release and thus the kinetics of hydration processes. When only cement is subjected to additional grinding (80FA/20PC(30)), the cement activity is improved which is revealed by shortening of the induction period and an increase in the intensity of the second heat release effect. On the contrary, when only fly ash is activated (80FA(30)/20PC) the duration of induction period is almost the same as in the case of non-activated mixture (80FA/20PC-0), the intensity of the second heat release stage is not improved, it is even reduced, while there is an additional effect of heat release at about 37 h (aluminate peak). In the case of individual grinding of fly ash and cement (80FA(30)/20PC(30)), the duration of the induction period is a bit shorter compared to that recorded for reference non-activated mixture. Moreover, the intensity of the second effect of heat release for 80FA(30)/20PC(30) mixture is the same as in the case of sample in which only cement was activated. Additional third effect of heat release is also observed for 80FA(30)/20PC(30) but it appears a few hours earlier (at about 30 h) than for 80FA(30)/20PC. Finally, the most intense effect of mechanical activation is seen when both components of the cement paste are ground together (80FA/20PC-30): a significant increase in the intensity of the exothermic effects related to the precipitation of the C–S–HFootnote 1 phase and hydration of the aluminates takes place. Total heat released after 48 h of hydration also depends on the way of mechanical activation and decreases as follows: 80FA/20PC-30 > 80FA/20PC(30), 80FA(30)/20PC(30) > 80FA/20PC-0 > 80FA(30)/20PC. Thus, the results show that in the case of individually milled components of fly ash–cement mixtures, activation of cement plays the main role in the intensity of hydration. However, when both components are activated concomitantly intense synergistic effect is observed.
Figure 7 presents the influence of the time of mechanical activation by the use of joint grinding on the rate of heat release and cumulative heat released for fly ash–cement pastes. It can be seen that even a short time of mechanical activation (5 min., 80FA/20PC-5) affects the kinetics of hydration processes: the course of calorimetric curve till about 26th h is similar to the one registered for the reference, then additional effect of heat release (aluminate peak) starts to appear; the cumulative heat released is initially lower compared to the result for non-activated paste and after 32 h slightly exceeds it.
Observed changes in the course of calorimetric curves and the cumulative heat released are the effect of changes in morphology of the grains and their reactivity due to the grinding process. Mechanical activation makes the grains of fly ash and cement comminuted and their outer layer is damaged which helps in improving their reactivity in the presence of water. In the case where the components were ground concomitantly, the cement grains could obtain a greater fineness compared to separate grinding because the harder fly ash grains acted as an additional fine abrasive agent. As a result, the paste performed with interground binder shows highest rate of heat evolution compared to the specimen containing separately milled ingredients. The total heat released after 48 h is also significantly higher in such case. It proves the acceleration of dissolution–precipitation processes in the activated fly ash–cement mixtures. A detailed discussion of the mechanism of hydration of the activated fly ash–cement blends in the first hours after adding water is presented below.
In the first period of hydration, cement and fly ash grains are wetted, some of their components are dissolved, calcium and sulphate ions go into solution and ettringite is formed within a few minutes after adding water [44].
During the induction period, in which the rate of reaction is slow, the concentration of Ca2+, evolved during hydration of alite, increases as long as the critical supersaturation is reached [40]. In the presence of fly ash, Ca2+ ions can be adsorbed on the surface of its grains [45]. As a result the amount of these ions in the solution is reduced which enhances dissolution of alite and its hydration. It can be expected that these processes proceed with greater intensity for mechanically activated samples. On the other hand, aluminate ions, introduced together with fly ash, work differently in relation to alite hydration, i.e.: retardation of alite hydration and hindering of C–S–H nucleation take place [41, 43, 44]. This is because even a small amount of aluminate ions in the solution can strongly inhibit the dissolution of alite by bonding of aluminate ions on the silicate grains. As a result, condensation of aluminosilicate species on the alite surface occurs [46, 47]. The used fly ash is rich in alumina. Due to the rupture of fly ash grains by mechanical activation, more aluminate ions can be released into pore solution [9]. This may be the reason of small extension of the induction period, reduction in the intensity of the next stage of heat releasing and also decrease of the cumulative heat evolved in the case of mixture in which only fly ash was activated (80FA(30)/20PC, Fig. 6). Also in the case of sample subjected to intergrinding for 5 min. (80FA/20PC-5, Fig. 7), the initial decrease in the amount of heat released is visible, however, in comparison with the sample 80FA(30)/20PC, this effect is compensated by the simultaneous activation of the cement. The above conclusions were also confirmed by the results obtained for the mixture composed of individually milled fly ash and cement (80FA(30)/20PC(30)). For this sample, the induction period is longer compared to the results for mixture in which only cement was activated (80FA/20PC(30)) but the next exothermic effect is not reduced. The described processes explain the small effect of the grinding on the induction period, despite the expected improvement in binder activity due to mechanical activation. Only in the case of long times of intergrinding, small reduction in the induction period is observed.
After the end of the induction period, a precipitation of Ca(OH)2 and C–S–H takes place, which intensifies with longer time of mechanical activation. To be precise, in the presence of aluminate ions, some amount of C–A–S–H phase (hydrated calcium aluminosilicates) is also created by the formation of Si–O–Al bonds [41, 44]. However, after the induction period, hindering of alite hydration is not further observed. This is confirmed by the fact that the intensity of silicate peak on the calorimetric curve is not reduced with the time of mechanical activation (Fig. 7) despite the initial retardation of alite hydration in the presence of aluminate ions. Precipitation of solid products makes that the ions are removed from the solution, and the dissolution process of alite is intensified [40]. It is conspicuous that the longer time of mechanical activation, the steeper the slope of the curve in the acceleration period (Fig. 7). It indicates that in the case of adequate comminution of the mixture, after reaching supersaturation of the solution at the end of the induction period, the physicochemical processes of precipitation of solid hydration products occur more rapidly and the time to form a solid structure is shorter compared to samples ground for a shorter time. An intense precipitation of hydrated silicate products results in adsorption of non-bound sulphate ions on C-S–H surface [41, 44, 46]. But after the depletion of gypsum, what is visible in our study, the hydration of aluminates is more intense which is confirmed in calorimetric curves by the appearance of aluminate peak. For example, it can be seen that the aluminate peak is observed a few hours earlier for 80FA(30)/20PC(30) than for 80FA(30)/20PC (Fig. 6) probably as a result of more intense precipitation process and faster depletion of sulphate in the case of sample containing mechanically activated fly ash and cement. In this case, not only the aluminate ions from the fly ash but also the aluminates from the cement are more easily available due to mechanical activation. Enriching the mixture with aluminates from cement causes the sulphate depletion point to be reached faster.
Another phenomenon favouring the precipitation of solid products is the effect of fly ash grains as nucleation centres for precipitation of C–S–H which in result enhances the rate of hydration of alite. This process is more efficient with smaller fly ash particles which is confirmed by steeper curve in the acceleration period. Thus, the longer time of mechanical activation, the more intense the acceleration effect on the calorimetric curve, also the aluminate peak which appears earlier is more intense. It indicates intensification of C–S–H/C–A–S–H precipitation but also enhanced dissolution of aluminates due to mechanical damage to the grains of the mixture. In the case of time of intergrinding longer than 40 min., the effect of precipitation of C–S–H phase and the aluminate effect are no longer distinguishable (Fig. 7). In such case, sulphate depletion point happens just after or overlaps with the peak of hydration of alite. Higher amount of aluminate ions in the solution makes it possible for a faster consumption of sulphate ions. It is visible that the longer the time of activation, the smaller and more irregular fly ash grains are and the aluminate effect appears faster and with higher intensity. Thus, the more intense mechanical activation, the more aluminate ions are able to go into solution and the system is undersulphated.
Regarding the enrichment of the pore water with silicate ions derived from fly ash, one can notice that this may result in formation of C–S–H phase of low Ca/Si ratio which is a known effect observed in the case of C–S–H phase formed in the presence of supplementary cementitious materials [48, 49]. Reduction in the concentration of Ca2+ ions by their higher adsorption on milled fly ash grains can also contribute to this effect. Narmluk et al. [45] indicated that the C–S–H of lower Ca/Si ratio precipitates in initial periods of hydration, and then transforms to more stable form in slow process. They concluded that this phenomenon could partially explain the lengthening of the induction period and slowing down of early hydration often observed for fly ash–cement slurries.
Basing on the calorimetric results it can be assumed that in the case of a sufficiently long mechanical activation time (more than 40 min. of intergrinding), the initial and final setting times are shortened compared to the reference. This is indicated by the shortening of the induction period and a significant steeper slope of the calorimetric curve in the next stage, as well as a shift of the maximum of the acceleration peak to the earlier hours of hydration. On the other hand, the shape of calorimetric curve indicates that in the case of long-time mechanical activation the system is undersulphated.
The results confirm that intergrinding is the more effective way of mechanical activation of VHVFA binder compared to separate milling. However, it should be noted that the complexity of investigated systems makes that hydration processes and consequently the properties of fresh and final composite depend not only on the intensity of activation. Other factors such as: the amount of fly ash, the curing temperature and water to cement ratio also influence the rate of hydration [45].
Based on the analysis of calorimetric tests, the mixtures interground for short and long time, i.e. 10 min. and 60 min. (80FA/20PC-10 and 80FA/20PC-60) were selected for further detailed studies of the influence of mechanical activation on phase composition and microstructure of the hardened material.
Thermal analysis
TG and DTG curves registered for fly ash–cement pastes after different periods of hydration are presented in Fig. 8, while Fig. 9 depicts mass losses at selected temperature ranges.
Comparison of the TG/DTG curves registered after 3 h of hydration and those for dry mixtures (0 h of hydration) shows that the first physicochemical changes took place during this short period after addition of water. The effect resulting from Ca(OH)2 decomposition is not visible because small amounts that were present in the dry mixtures have undergone reaction and new parts have not precipitated yet. This is not surprising because after 3 h of hydration all the tested mixtures are still in the induction period. At the temperature range till 150 °C, two mass losses are visible being the effect of dehydration of the first products of hydration (ettringite mainly) and unreacted gypsum. It is visible that mass loss registered at this temperature range, especially the mass loss related to the first step on the TG curve, rises with longer grinding time.
After 24 h of hydration the presence of unreacted gypsum is no longer evident in all tested samples. One effect with the extremum near 100 °C is visible on DTG curves in the temperature range discussed above. It is the effect of dehydration of ettringite and C–S–H phase. The mass loss at 390–450 °C appears. It results from the decomposition of Ca(OH)2 and confirms the presence of this hydration product in the fly ash–cement pastes. This mass loss increases with the hydration time until 7th day, then it declines sharply on 28th day (Fig. 9b). On 90th day of hydration, presence of Ca(OH)2 is not detected in the TG and DTG curves (Fig. 8). This shows that in the first days after adding water to the mixtures, cement hydration prevails over the pozzolanic reaction. In the following days, the pozzolanic activity of fly ash develops and Ca(OH)2 reacts with active ingredients of fly ash, creating an additional amount of C–S–H and C–A–S–H products until the Ca(OH)2 is completely depleted. Also mass loss relating to decomposition of CaCO3 (at 550–730 °C) reduces starting from the 28th day which indicates that this component of fly ash–cement paste undergoes chemical transformation over time.
Starting from 3rd day of hydration the effect on DTG curves near 150 °C becomes gradually visible (Fig. 8). It indicates the formation of hydrated aluminate phases, e.g. carboaluminates. On 3rd day of hydration, this effect is hardly visible and only for the sample 80FA/20PC-60. In later days of hydration, it is clearly visible for all mixtures, but still more intense for activated samples compared to the reference.
In general, the mass loss up to 390 °C, i.e. until the decomposition of Ca(OH)2, can be regarded as water bound in hydrates. Dehydration of the various hydration products, taking place one after the other and also overlapping, occurs in this temperature range. This mass loss increases over time for all samples as a result of the processes of cement hydration and in later periods also the pozzolanic reaction (Fig. 9a). One can see that this effect is higher for the activated pastes compared to the reference, thus more water is bound. Until 7th day, the amount of bound water is the highest for 80FA/20PC-60, then it equalizes to the result for the sample activated for 10 min.
Interesting conclusions can be drawn after analysing the mass loss at 390–450 °C (Fig. 9b). This loss in mass is proportional to the content of Ca(OH)2 in the sample. The shape of the DTG curves shows that this component is absent both in the 3 h of hydration and after 90 days of hydration. Thus, especially after 90th day, the small mass loss read from the TG curves corresponds to the continuous dehydration of other aluminosilicate products of hydration. As it was mentioned earlier, the amount of Ca(OH)2 increases in early days of hydration with longer the grinding time. This confirms that mechanical activation accelerates the precipitation of cement hydration products after the induction period, as observed in calorimetric results. The results registered on 3rd and 7th day are very similar, whereas only between the 7th and 28th day there is a clear reduction in the amount of the hydroxide, unambiguously confirming that the pozzolanic reaction is taking place. However, increasing amount of bound water during the discussed period and slight changes in the amount of Ca(OH)2 indicate that the pozzolanic reaction probably started earlier, that is between 3rd and 7th day of hydration. It should also be noted that on 7th day the amount of Ca(OH)2 was still higher for activated samples compared to the reference. However, the degree of reduction of Ca(OH)2 on 28th day is also greater for the activated samples, which confirms that grinding improves the pozzolanic activity. This improvement is more pronounced with longer grinding time.
In summary, the results of thermal analysis show that mechanical activation accelerates the hydration of cement and makes the pozzolanic reaction more intense. Hydrated aluminate phases appear earlier in the mechanically activated mixtures.
X-ray diffraction
The X-ray diffraction patterns are presented in Fig. 10. The results show that mechanical activation does not influence the kinds of crystalline products formed in the fly ash–cement pastes. In each tested hydration period, the presence of weakly reactive crystalline components of fly ash, i.e. quartz and mullite, is visible in the samples. On the 1st day, fly ash–cement pastes contained alite which had not reacted during the short hydration period. This component disappears completely over time. The appearance of Ca(OH)2 and ettringite is the proof of cement hydration. On the other hand, the disappearance of Ca(OH)2 over time confirms pozzolanic activity of fly ash. The X-ray diffraction patterns registered after 28th day of hydration show formation of carboaluminate phases. One can see that in the case of the mixture mechanically activated for 60 min., the reaction between CaCO3 and aluminates was amplified. This is because of better availability of aluminate ions to the reaction and increased activity of CaCO3 due to grinding. It should be noted that the carboaluminate formation reaction can also involve Ca(OH)2, thus it also contributes to the reduction of Ca(OH)2 content, but only to a minor extent.
The presence of ettringite in the specimens is visible not only on the early hydration period but also after 90 days. This indicates that there are conditions that stabilize this sulfoaluminate phase. Transformation of ettringite to monosulphate is prevented and formation of carboaluminate is preferred.
FTIR spectroscopy
FTIR spectra (Fig. 11) registered after different periods of hydration of the fly ash–cement pastes gradually change over time. One can see that the band relating to stretching vibrations of water molecules (3200–3700 cm−1) increases in intensity while the position of the band of bending vibrations (at about 1630 cm−1) moves to slightly higher wavenumbers due to the formation of hydration products. Moreover, small additional effects, changing over time, may be separated at the broad band of stretching water vibrations. The changes indicate formation of different products of hydration. For example, the effects at about 3420 cm−1 and about 3635 cm−1 come probably from the vibration of H2O and OH in ettringite [36] and they can overlap with the bands typical for gypsum (at about 3550 and 3400 cm−1 [36]).
The presence of Ca(OH)2 (the band about 3640 cm−1) is hardly visible, only as a shoulder. This is due to low amount of the component in the samples and overlapping the bands situated in this region.
The main broad band of stretching vibrations of Si(Al)–O bonds (at about 1085 cm−1) shifts its extremum over time towards lower wavenumbers which is a sign of the formation of new silicate and aluminosilicate phases as a result of hydration reaction. One can see that during the first 3 days this displacement is more pronounced for the activated samples, thus early hydration is accelerated in those cases. Then, after 7th day of hydration, visible effect at about 970 cm−1 appears which confirms creation of C–S–H phase.
After 3 days, poorly visible band at about 425 cm−1 is starting to appear. It may be related to Al-O vibrations [36, 50] in newly created phases of hydrated aluminates and aluminosilicates. Another small intensity bands at about 670 and 735 cm−1, appearing at later periods of hydration, also confirm formation of hydrated phases such as C–S–H and C–A–S–H.
The band typical for carbonates, i.e. at about 1425 cm−1, changes its shape over time. This indicates that these components also undergo chemical reactions.
SEM
The results of SEM analysis after 28 days of hydration were presented in Figs. 12–14. It can be seen that, in general, the hardened matrix consists of unreacted fly ash grains bound together by the hydration products. In the case of mechanically activated binder for 60 min., the hardened matrix is more compact compared to the reference.
C–S–H phase in forms similar to honeycomb structure was created between and on the fly ash grains. In much smaller amounts the ettringite rods are also visible. There are also aluminate or aluminosilicate hydrates created in the form of plates visible in the case of mechanically activated mixtures (Fig. 13).
Conclusions
-
1.
Mechanical activation of fly ash–cement mixtures results in significant comminution of the grains. Very small grains of fly ash cannot be further crushed and remain in unchanged form in the system. There is a grinding time limit beyond which further reduction in grain size is insignificant. Grinding for 60 min. results that almost 100% of the grains have the desirable diameter below 45 μm.
-
2.
Concomitant grinding of cement and fly ash is more effective compared to separate grinding of these materials. It was found that such kind of activation does not cause significant changes in the phase composition of the dry VHVFA binder. Thus, the diameter of grains, their specific surface area and damage of outer layers play the main roles in the activating process.
-
3.
Mechanical activation modifies kinetics of hydration processes. Damage of fly ash grains results in better availability of aluminate ions that can delay early hydration of alite. Despite that, in the case of concomitant grinding, precipitation of C–S–H/C–A–S–H phase after induction period is enhanced and cement hydration is accelerated.
-
4.
Pozzolanic activity of fly ash in the blend obtained by concomitant grinding is improved which is especially visible after 7th day of hydration. Longer milling time favors the reduction of Ca(OH)2 in the pozzolanic reaction. On the 90th day of hydration, the presence of Ca(OH)2 is no longer observed in the samples, regardless of the activation process.
-
5.
Mechanical activation promotes the formation of hydrated aluminate and aluminosilicate products, including the carboaluminates. The conversion of ettringite to monosulphate is slowed down so that ettringite is present in all the samples even after 90 days of hydration.
-
6.
Mechanical activation and its time affect the quantitative composition of fly ash–cement pastes, and only slightly the qualitative composition. In the case of binder mechanically activated for 60 min., the hardened matrix is more compact compared to the reference.
-
7.
Mechanical activation by concomitant grinding of cement and fly ash is more effective in the case of longer time of milling. Basing on the calorimetric results one may conclude that the time of grinding more than 40 min. may be sufficient.
-
8.
Comparing the results obtained in this work with those presented in Part 1 [26] confirms that the mechanisms of mechanical and chemical activation are different. In general, mechanical activation primarily results in faster physical availability of components for reaction and is of greatest importance in the first periods of hydration, unlike chemical activation, where modification of the hydration process occurs throughout whole studied hydration period.
Notes
Abbreviations used in cement chemistry: C–CaO, A–Al2O3, S–SiO2, H–H2O; C–S–H–hydrated calcium silicates, the main product of cement hydration.
References
Igliński B, Buczkowski R. Development of cement industry in Poland—History, current state, ecological aspects. A review J Clean Prod. 2017;141:702–20.
Gartner E, Hirao H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem Concr Res. 2015;78:126–42.
Andrew RM. Global CO2 emissions from cement production. Earth Syst Sci Data. 2018;10:195–217.
CEMBUREAU—The European cement association; SPC—Stowarzyszenie Producentów Cementu. Rola Cementu w Niskoemisyjnej Gospodarce do Roku 2050. https://lowcarboneconomy.cembureau.eu/wp-content/uploads/2018/09/Gospodarka-niskoemisyjna-2050-Sektor-cementowy.pdf. Accessed 27 February 2023.
Liew KM, Sojobi AO, Zhang LW. Green concrete: Prospects and challenges. Constr Build Mater. 2017;156:1063–95.
Wilińska I, Pacewska B. Influence of selected activating methods on hydration processes of mixtures containing high and very high amount of fly ash—a review. J Therm Anal Calorim. 2018;133:823–43.
Mucsi G. Mechanical activation of power station fly ash by grinding—A review. J Silicate Based Compos Mater. 2016;68:56–61.
Sekulić Ž, Popov S, Ouričić M, Rosić A. Mechanical activation of cement with addition of fly ash. Mater Lett. 1999;39:115–21.
Kato K, Xin Y, Hitomi T, Shirai T. Surface modification of fly ash by mechano-chemical treatment. Ceram Int. 2019;45:849–53.
Kumar S, Kumar R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram Int. 2011;37:533–41.
Guo X, Xiang D, Duan G, Mou P. A review of mechanochemistry applications in waste management. Waste Manag. 2010;30:4–10.
Abadi AG, Al-Shandoudi L. Fly ash morphology and surface modification via mechanical activation: a review. Environ Conserv J. 2020;21:105–12.
Paul KT, Satpathy SK, Manna I, Chakraborty KK, Nando GB. Preparation and characterization of nano structured materials from fly ash: a waste from thermal power stations, by high energy ball milling. Nanoscale Res Lett. 2007;2:397–404.
Cristelo N, Tavares P, Lucas E, Miranda T, Oliveira D. Quantitative and qualitative assessment of the amorphous phase of a Class F fly ash dissolved during alkali activation reactions—Effect of mechanical activation, solution concentration and temperature. Compos B. 2016;103:1–14.
Bondar D, Coakley E. Effect of grinding on early age performance of high volume fly ash ternary blended pastes with CKD & OPC. Constr Build Mater. 2017;136:153–63.
Marjanović N, Komljenović M, Baščarević Z, Nikolić V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr Build Mater. 2014;57:151–62.
Du S, Zhao Q, Shi X. High-volume fly ash-based cementitious composites as sustainable materials: an overview of recent advances. Advances in Civil Engineering, 2021, Article ID 4976169; https://doi.org/10.1155/2021/4976169
Patil AG, Anandhan S. Ball milling of class-f indian fly ash obtained from a thermal power station. Int J Energy Eng. 2012;2:57–62.
Babu Rao J, Narayanaswami P, Siva PK. Thermal stability of nano structured fly ash synthesized by high energy ball milling. Int J Eng Sci Technol. 2010;2:284–99.
You I, Yoo D-Y, Doh J-H, Zi G. Performance of glass-blended cement produced by intergrinding and separate grinding methods. Cem Concr Compos. 2021;118:103937.
Ghiasvand E, Ramezanianpour AA, Ramezanianpour AM. Effect of grinding method and particle size distribution on the properties of Portland-pozzolan cement. Constr Build Mater. 2014;53:547–54.
Erdogdu K, Tokyay M, Türker P. Comparison of intergrinding and separate grinding for the production of natural pozzolan and GBFS-incorporated blended cements. Cem Concr Res. 1999;29:743–6.
El Fami N, Ez-zaki H, Boukhari A, Khachani N, Diouri A. Influence of mechanical activation of fly ash on the properties of Portland cement mortars. Mater Today: Proc. 2022;58:1419–22.
Aydın S, Karatay Ç, Baradan B. The effect of grinding process on mechanical properties and alkali–silica reaction resistance of fly ash incorporated cement mortars. Powder Technol. 2010;197:68–72.
Kumar R, Kumar S, Mehrotra SP. Towards sustainable solutions for fly ash through mechanical activation. Resour Conserv Recycl. 2007;52:157–79.
Wilińska I, Pacewska B, Ostrowski A. Investigation of different ways of activation of fly ash–cement mixtures. Part 1. Chemical activation by Na2SO4 and Ca(OH)2. J Therm Anal Calorim. 2019;138:4203–13.
Wilińska I, Pacewska B. Zastosowanie kalorymetrii we wstępnych badaniach aktywowanych mieszanek popiołowo-cementowych. Przem Chem. 2017;96:761–5 ((in Polish)).
Pacewska B, Wilińska I, Blonkowski G. Investigations of cement early hydration in the presence of chemically activated fly ash. Use of calorimetry and infrared absorption methods. J Therm Anal Calorim. 2008;93:769–76.
Poznański J. Computer software for processing of data obtained from calorimeter, 2012.
Payá J, Monzó J, Borrachero MV, Perris E, Amahjour F. Thermogravimetric methods for determining carbon content in fly ashes. Cem Concr Res. 1998;28:675–86.
van der Merwe EM, Strydom CA, Potgieter JH. Thermogravimetric analysis of the reaction between carbon and CaSO4⋅2H2O, gypsum and phosphogypsum in an inert atmosphere. Thermochim Acta. 1999;340–341:431–7.
Wilińska I, Pacewska B. Comparative investigation of reactivity of different kinds of fly ash in alkaline media. J Therm Anal Calorim. 2019;138:3857–72.
Fernández-Jiménez A, Palomo A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005;86:207–14.
Criado M, Fernádez-Jiménez A, Palomo A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio Part I: FTIR study. Microporous Mesoporous Mater. 2007;106:180–91.
Mozgawa W, Król M, Dyczek J, Deja J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;132:889–94.
Fernández-Carrasco L, Torrens-Martín D, Morales LM, Martínez-Ramírez S. Infrared spectroscopy in the analysis of building and construction materials. Infrared Spectrosc Mater Sci Eng Technol InTech. 2012;369–382. https://www.intechopen.com/books/infrared-spectroscopy-materials-science-engineering-andtechnology/infrared-spectroscopy-of-cementitious-materials. Accessed 28 February 2023.
Li H, Chen Y, Cao Y, Liu G, Li B. Comparative study on the characteristics of ball-milled coal fly ash. J Therm Anal Calorim. 2016;124:839–46.
Patil AG, Anandhan S. Influence of planetary ball milling parameters on the mechano-chemical activation of fly ash. Powder Technol. 2015;281:151–8.
Irbe L, Urbonas L, Heinz D. Coal fly ash activation—Comparison of isothermal calorimetric data and mortar strength. Thermochim Acta. 2018;659:151–6.
Scrivener KL, Juilland P, Monteiro PJM. Advances in understanding hydration of Portland cement. Cem Concr Res. 2015;78:38–56.
Bergold ST, Goetz-Neunhoeffer F, Neubauer J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem Concr Res. 2017;93:32–44.
Hu J, Ge Z, Wang K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr Build Mater. 2014;50:657–63.
Schöler A, Lothenbach B, Winnefeld F, Haha MB, Zajac M, Ludwig H-M. Early hydration of SCM-blended Portland cements: a pore solution and isothermal calorimetry study. Cem Concr Res. 2017;93:71–82.
Neto JDSA, Angeles G, Kirchheim AP. Effects of sulfates on the hydration of Portland cement–A review. Const Build Mater. 2021;279:122428.
Narmluk M, Nawa T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem Concr Res. 2011;41:579–89.
Nicoleau L, Schreiner E, Nonat A. Ion-specific effects influencing the dissolution of tricalcium silicate. Cem Concr Res. 2014;59:118–38.
Pustovgar E, Mishra RK, Palacios M, d’Espinose de Lacaillerie J-B, Matschei T, Andreev AS, Heinz H, Verel R, Flatt RJ. Influence of aluminates on the hydration kinetics of tricalcium silicate. Cem Concr Res. 2017;100:245–62.
Lothenbach B, Scrivener K, Hooton RD. Supplementary cementitious materials. Cem Concr Res. 2011;41:1244–56.
Rossen JE, Lothenbach B, Scrivener KL. Composition of C-S–H in pastes with increasing levels of silica fume addition. Cem Concr Res. 2015;75:14–22.
Trezza MA, Lavat AE. Analysis of the system 3CaO⋅Al2O3-CaSO4⋅2H2O-CaCO3-H2O by FT-IR spectroscopy. Cem Concr Res. 2001;31:869–72.
Author information
Authors and Affiliations
Contributions
IW conceptualization; methodology, investigation, formal analysis, writing—original draft preparation, writing—review and editing. BP conceptualization, formal analysis, writing—review and editing. AO investigation, formal analysis.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilińska, I., Pacewska, B. & Ostrowski, A. Investigation of different ways of activation of fly ash–cement mixtures: part 2—mechanical activation. J Therm Anal Calorim (2023). https://doi.org/10.1007/s10973-023-12503-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-023-12503-8