Abstract
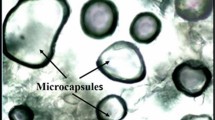
In this study, cetyl alcohol (CA) (CH3(CH2)15OH), which is a fatty alcohol, was microencapsulated by the coacervation method with the titanium-n-butoxide (TNBO). Firstly, at the beginning of the experimental process by thermal and structural analyses, the CA was determined as having a phase change material (PCM) property, then it was microencapsulated with TNBO. In the second step of the study, barite, boron nitride, marble powder, and fly ash were separately added to prepare a series of microencapsulated with TNBO. Lastly, structural and thermal properties of both microencapsulated PCM and composite supported microencapsulated PCMs (CSM-PCMs) have been analyzed by FTIR, DSC, TGA, and SEM devices. Surprisingly, the SEM image of CA turned from torn fiber structure into a particulate structure in the form of groundnut in the SEM images of the microencapsulated CA-Ti taken at different magnifications. After the thermal cycling test, the same analyses have been made before and after thermal cycling to compare the structural and thermal properties. It has been investigated whether to use as insulation material. It is concluded that the most suitable samples, compared with structural and thermal properties, are barite (B) and boron nitride (BN) with microencapsulated samples. The obtained results are shown that CATi-B and CATi-BN are CSM-PCMs and could be suggested in terms of thermal energy storage in buildings as promising PCMs.



















Similar content being viewed by others
References
Trivedi G, Parameshwaran R. Microencapsulated phase change material suspensions for cool thermal energy storage. Mater Chem Phys. 2020;242:122519. https://doi.org/10.1016/j.matchemphys.2019.122519.
Genc ZK, Canbay CA, Acar SS, Sekerci M, Genc M. Preparation and thermal properties of heterogeneous composite phase change materials based on camphene–palmitic acid. J Therm Anal Calorim. 2015;120(3):1679–88. https://doi.org/10.1007/s10973-015-4478-3.
Feczkó T, Trif L, Horák D. Latent heat storage by silica-coated polymer beads containing organic phase change materials. Sol Energy. 2016;132:405–14. https://doi.org/10.1016/j.solener.2016.03.036.
Qi C, Zhang F, Mu J, Zhang Y, Yu Z. Enhanced mechanical and thermal properties of hollow wood composites filled with phase-change material. J Clean Prod. 2020;256:120373. https://doi.org/10.1016/j.jclepro.2020.120373.
Lin Y, Jia Y, Alva G, Fang G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew Sustain Energy Rev. 2018;82:2730–42. https://doi.org/10.1016/j.rser.2017.10.002.
Veerakumar C, Sreekumar A. Preparation, thermophysical studies, and corrosion analysis of a stable capric acid/cetyl alcohol binary eutectic phase change material for cold thermal energy storage. Energy Technol. 2018;6(2):397–405. https://doi.org/10.1002/ente.201700540.
Huang X, Alva G, Liu L, Fang G. Microstructure and thermal properties of cetyl alcohol/high density polyethylene composite phase change materials with carbon fiber as shape-stabilized thermal storage materials. Appl Energy. 2017;200:19–27. https://doi.org/10.1016/j.apenergy.2017.05.074.
Ayaz H, Chinnasamy V, Cho H. Characterization and reliability of caprylic acid-stearyl alcohol binary mixture as phase change material for a cold energy storage system. Mater. 2021;14(23):7418. https://doi.org/10.3390/ma14237418.
Liu H, Wang X, Wu D, Ji S. Fabrication and applications of dual-responsive microencapsulated phase change material with enhanced solar energy-storage and solar photocatalytic effectiveness. Sol Energy Mater Sol Cells. 2019;193:184–97. https://doi.org/10.1016/j.solmat.2019.01.012.
Li M, Liu J, Shi J. Synthesis and properties of phase change microcapsule with SiO2-TiO2 hybrid shell. Sol Energy. 2018;167:158–64. https://doi.org/10.1016/j.solener.2018.04.016.
Sánchez L, Sánchez P, de Lucas A, Carmona M, Rodríguez JF. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym Sci. 2007;285(12):1377–85. https://doi.org/10.1007/s00396-007-1696-7.
Feczkó T, Trif L, Németh B, Horák D. Silica-coated poly (glycidyl methacrylate-ethylene dimethacrylate) beads containing organic phase change materials. Thermochim Acta. 2016;641:24–8. https://doi.org/10.1016/j.tca.2016.08.016.
Shah KW. A review on enhancement of phase change materials-A nanomaterials perspective. Energy Build. 2018;175:57–68. https://doi.org/10.1016/j.enbuild.2018.06.043.
Genc M, Karagoz GZ. Microencapsulated myristic acid–fly ash with TiO2 shell as a novel phase change material for building application. J Therm Anal Calorim. 2018;131(3):2373–80. https://doi.org/10.1007/s10973-017-6781-7.
Dubey R. Microencapsulation technology and applications. Def Sci J. 2009;59(1):82–95. https://doi.org/10.14429/dsj.59.1489.
Binici H, Gemci R, Kucukonder A, Solak HH. Investigating sound insulation, thermal conductivity and radioactivity of chipboards produced with cotton waste, fly ash and barite. Constr Build. 2012;30:826–32. https://doi.org/10.1016/j.conbuildmat.2011.12.064.
Berardi U, Gallardo AA. Properties of concretes enhanced with phase change materials for building applications. Energy Build. 2019;199:402–14. https://doi.org/10.1016/j.enbuild.2019.07.014.
Jeong S-G, Lee J-H, Seo J, Kim S. Thermal performance evaluation of bio-based shape stabilized PCM with boron nitride for energy saving. Int J Heat Mass Transf. 2014;71:245–50. https://doi.org/10.1016/j.ijheatmasstransfer.2013.12.017.
Li J-S, Zhang C-R, Li B, Cao F, Wang S-Q. Boron nitride coatings by chemical vapor deposition from borazine. Surf Coat Technol. 2011;205(12):3736–41. https://doi.org/10.1016/j.surfcoat.2011.01.032.
Yang J, Tang L-S, Bao R-Y, Bai L, Liu Z-Y, Yang W, et al. Largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem Eng J. 2017;315:481–90. https://doi.org/10.1016/j.cej.2017.01.045.
Jia X, Li Q, Ao C, Hu R, Xia T, Xue Z, et al. High thermal conductive shape-stabilized phase change materials of polyethylene glycol/boron nitride@ chitosan composites for thermal energy storage. Compos-A Appl Sci Manuf. 2020;129:105710. https://doi.org/10.1016/j.compositesa.2019.105710.
Yang J, Tang L-S, Bao R-Y, Bai L, Liu Z-Y, Xie B-H, et al. Hybrid network structure of boron nitride and graphene oxide in shape-stabilized composite phase change materials with enhanced thermal conductivity and light-to-electric energy conversion capability. Sol Energy Mater Sol Cells. 2018;174:56–64. https://doi.org/10.1016/j.solmat.2017.08.025.
Giro-Paloma J, Martínez M, Cabeza LF, Fernández AI. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): a review. Renew Sust Energ Rev. 2016;53:1059–75. https://doi.org/10.1016/j.rser.2015.09.040.
Canbay CA, Genc ZK, Acar SS, Sekerci M, Genc M. Preparation and thermodynamic properties of camphene/stearic acid composites as phase-change materials in buildings. Int J Thermophys. 2014;35(8):1526–37. https://doi.org/10.1007/s10765-014-1724-z.
Zhang W, Shi J, Wang X, Jiang Z, Song X, Ai Q. Conferring an adhesion layer with mineralization-inducing capabilities for preparing organic–inorganic hybrid microcapsules. J Mater Chem B. 2014;2(10):1371–8. https://doi.org/10.1039/C3TB21202E.
Marques JO, Bellato CR, de Souza CH, Silva RCD, Rocha PA. Synthesis, characterization and enhanced photocatalytic activity of iron oxide/carbon nanotube/Ag-doped TiO2 nanocomposites. J Braz Chem Soc. 2017;28:2301–12.
Karabulut S. Preparation of inorganic based microcapsules of cetyl alcohol and investigation of phase change material (FDM) behaviour: Master Thesis. Graduate School of Natural and Applied Sciences: Firat University; 2019.
Yin D, Ma L, Geng W, Zhang B, Zhang Q. Microencapsulation of n-hexadecanol by in situ polymerization of melamine–formaldehyde resin in emulsion stabilized by styrene–maleic anhydride copolymer. Int J Energy Res. 2015;39(5):661–7. https://doi.org/10.1002/er.3276.
Bu X, Zhou Y, He M, Chen Z, Zhang T. Optically active SiO2/TiO2/polyacetylene multilayered nanospheres: preparation, characterization, and application for low infrared emissivity. Appl Surf Sci. 2014;288:444–51. https://doi.org/10.1016/j.apsusc.2013.10.053.
Cao L, Tang F, Fang G. Preparation and characteristics of microencapsulated palmitic acid with TiO2 shell as shape-stabilized thermal energy storage materials. Sol Energy Mater Sol Cells. 2014;123:183–8. https://doi.org/10.1016/j.solmat.2014.01.023.
Soraya M, Abdel-Wahab F, Elamin A, Shaaban E, Ali Karrar N. Structural and thermal characteristics of Ge30− xSbxTe10Se60 (0≤ x≤ 20) glasses for electronic devices. J Therm Anal Calorim. 2023;1:1–16. https://doi.org/10.1007/s10973-023-12165-6.
Ouikhalfan M, Sarı A, Hekimoğlu G, Gencel O, Tyagi V. Thermal energy storage properties, thermal conductivity, chemical/and thermal reliability of three different organic phase change materials doped with hexagonal boron nitride. Surf Interfaces. 2022;1:102176. https://doi.org/10.1016/j.surfin.2022.102176.
Atinafu DG, Dong W, Wang J, Huang X, Wang J, Gao H, et al. Synthesis and characterization of paraffin/metal organic gel derived porous carbon/boron nitride composite phase change materials for thermal energy storage. Eur J Inorg Chem. 2018;2018(48):5167–75. https://doi.org/10.1002/ejic.201800811.
Acknowledgements
The Authors grateful to Firat University Scientific Research Projects Unit (FUBAP) for financial support with the project numbered (FF.17.32).
Author information
Authors and Affiliations
Contributions
SK contributed to investigation, formal Analysis, and writing. MS contributed to first idea, visualization, supervision, writing—review and editing. RA contributed to investigation, formal analysis, writing—review and editing, and visualization. ZKG contributed to formal analysis, visualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
This research has no animal experiments. So that, no ethical approval is required.
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karabulut, S., Sekerci, M., Adiguzel, R. et al. Preparation of composite supported phase change materials from inorganic-based microcapsules of cetyl alcohol and investigation of their phase change material (PCM) behaviors. J Therm Anal Calorim 148, 10679–10695 (2023). https://doi.org/10.1007/s10973-023-12433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12433-5