Abstract
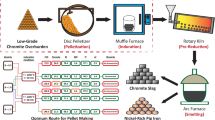
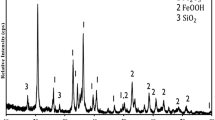
This paper focuses on the carbothermic reduction of red mud in the presence of sodium carbonate. The emphasis is on the reduction step in the ‘Elgai process’ which is central to the chemical beneficiation and carried out in the temperature range where fusing of roasting mass is avoided. The research, aimed at developing a fundamental understanding of the roasting has three different facets: (a) calculation of phase equilibrium in Fe2O3–Al2O3–Na2CO3–C and related systems for the relevant compositions and temperature range (650–950 °C); (b) simultaneous thermal analysis (TG/DTA) studies based on phase stability; and (c) roasting and leaching of red mud-soda-carbon pellets. Red mud was used in the as-received condition and after mechanical activation in an attrition mill. Red mud and roasted products were characterised by X-ray powder diffraction (XRD) and scanning electron microscopy (SEM) with X-ray microanalysis (EDS). The reduction temperature and carbon requirement were found to depend on the Al2O3 content of red mud, notably the occurrence of aluminogoethite. The interdependence of the decomposition of sodium carbonate, metallisation of red mud, sodium aluminate formation, and alumina recovery are elucidated. Mechanical activation improves the degree of metallisation and alumina recovery. The significance of Ca(OH)2 addition as a way forward to reduce the decomposition temperature of sodium carbonate and the consequent reduction in the roasting temperature are highlighted.



















Similar content being viewed by others
References
European Aluminium Association. Bauxite Residue Management : Best Practice. 2013 [cited 2021 Aug 8];32. Available from: http://bauxite.world-aluminium.org/fileadmin/_migrated/content_uploads/Bauxite_Residue_Management_-_Best_Practice__English_.pdf
Red mud [Internet]. 2021 [cited 2021 Aug 8]. Available from: https://en.wikipedia.org/wiki/Red_mud
Khairul MA, Zanganeh J, Moghtaderi B. The composition, recycling and utilisation of Bayer red mud. Resour Conserv Recycl. 2019;141:483–98.
Jones BEH, Haynes RJ. Bauxite processing residue: a critical review of its formation, properties, storage, and revegetation. Crit Rev Environ Sci Technol. 2011;41:271–315.
Kumar R, Srivastava JP, Premchand. Utilization of iron values of red mud for metallurgical applications. Bandopadhyay A, Goswami NG, Rao PR, editors. Environ. Waste Manag. (ISSN 0971–9407). Jamshedpur - 831007, India: NML Jamshedpur (India); 1998. Available from: http://eprints.nmlindia.org/7364/
Technology Roadmap for Bauxite Residue Treatment and Utilization. The Aluminium Association, Washington DC, February 2000.
Anich I, Bagshaw T, Margolis N, Skillingberg M. The alumina technology roadmap. In: Donaldson D, Raahauge BE, editors. In: Essential readings in light metals. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-48176-0_13.
Cusano G, Rodrigo Gonzalo M, Farrell F, Remus R, Roudier S, Delgado Sancho L. Best Available Techniques (BAT) Reference Document for the Non-Ferrous Metals Industries - Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control). Ind. Emiss. Dir. 2010/75/EU Integr. Pollut. Prev. contro [Internet]. 2017 [cited 2018 Oct 18]; Available from: http://eippcb.jrc.ec.europa.eu/reference/BREF/NFM/JRC107041_NFM_bref2017.pdf
Rai S, Wasewar KL, Mukhopadhyay J, Yoo CK, Uslu H. Neutralization and utilization of red mud for its better waste management. Arch Environ Sci. 2012;6:13–33.
Swain B, Akcil A, Chun LJ. Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Crit Rev Environ Sci Technol. 2020;52:520–750.
Pontikes Y. Bauxite residue valorization and best practices: preface for the thematic section and some of the work to follow. J Sustain Metall. 2016;2:313–5.
Evans K. The history, challenges, and new developments in the management and use of bauxite residue. J Sustain Metall. 2016;2:316–31.
Tsesmelis K. Global overview of bauxite residue management & reuse [Internet]. 3rd Int. Bauxite Residue Valoris. Best Pract. Conf. - 29 Sept. 2020. 2020 [cited 2022 May 8]. Available from: https://conference2020.redmud.org/
Bayer KJ. Process of making alumina [Internet]. United State of America; 1894. Available from: https://patents.google.com/patent/US515895A/en
Prasad PM. Bauxite tailings (red muds) disposal management via utilisation. In: Ramachandrarao P, Kumar R, Srikanth S, Goswami NG, editors. Nonferrous Extr. Metall. New Millenn. National Metallurgical Laboratory, Jamshedpur (India); 1999. p. 385–410.
Samal S, Ray AK, Bandopadhyay A. Proposal for resources, utilization and processes of red mud in India — a review. Int J Miner Process. 2013;118:43–55.
Paramguru RK, Rath PC, Misra VN. Trends in red mud utilization – a review. Miner Process Extr Metall Rev. 2004;26:1–29.
Piga L, Pochetti F, Stoppa L. Recovering metals from red mud generated during alumina production. J Met. 1993;45:54–9.
Thakur RS, Das SN. Red mud analysis and utilisation and recovery of metal values. New Delhi: Publications and Information Directorate (CSIR) and Wiley Eastern Limited; 1994.
Andrejcak M, Soucy G. Patent review of red mud treatment – product of Bayer process. Acta Metall Slovaca. 2004;10:347–68.
Bonomi C, Cardenia C, Yin PTW, Panias D. Review of technologies in the recovery of iron, aluminium, titanium and rare earth elements from bauxite residue (Red Mud). 3rd Int. Enhanc. Landfill Min. | Lisbon – Port. | 8th – 10th Febr. 2016. 2016. p. 259–76.
Hammond K, Mishra B, Apelian D, Blanpain B. CR3 communication: red mud – a resource or a waste? J Met. 2013;65:340–1.
Jamieson EJ. Development and utilisation of Bayer process by-products [Internet]. Curtin University, School of Civil and Mechanical E; 2014. Available from: http://espace.library.curtin.edu.au/R?func=dbin-jump-full&local_base=gen01-era02&object_id=197113
Kitajima T, Kasai E. Carbothermic reduction of bauxite residue. Shigen-to-Sozai. 1999;115:611–7.
Klauber C, Gräfe M, Power G. Bauxite residue issues: II options for residue utilization. Hydrometallurgy. 2011;108:11–32.
Kumar S, Kumar R, Bandopadhyay A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour Conserv Recycl. 2006;48:301–14.
Mishra B, Gostu S. Materials sustainability for environment: Red-mud treatment. Front Chem Sci Eng. 2017;11:483–96.
Hertel T, Pontikes Y. Geopolymers, inorganic polymers, alkali-activated materials and hybrid binders from bauxite residue (red mud) – Putting things in perspective. J Clean Prod. 2020;258: 120610.
Liu Y, Naidu R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014;34:2662–73.
Liu Z, Li H. Metallurgical process for valuable elements recovery from red mud—a review. Hydrometallurgy. 2015;155:29–43.
Pontikas Y. No Title No Title. In: Pontikes Y, editor. Bauxite Residue Valoris. Best Pract. Conf. 5–7 October, 2015, Leuven. Leuven; 2015.
Pontikes Y, Angelopoulos GN. Bauxite residue in cement and cementitious applications: Current status and a possible way forward. Resour. Conserv. Recycl. 2013. p. 53–63.
Reid S, Tam J, Yang M, Azimi G. Technospheric mining of rare earth elements from bauxite residue (red mud): process optimization, kinetic investigation, and microwave pretreatment. Sci Reports. 2017;7:1–9.
Balomenos E, Panias D, Pontikes Y. MUD2METAL: A holistic flow sheet for the bauxite residue valorisation. Bauxite Residue Valoris. Best Pract. Conf. 5–7 October, 2015, Leuven. 2015. p. 129–36.
Balomenos E, Gianopoulou I, Panias D, Paspaliaris I, Boufounos D, Perry K. Efficient and complete exploitation of the Bauxite Residue (Red Mud) produced in the Bayer process. Proc - Eur Metall Conf EMC. 2011;2011:745–58.
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall. 2016;2:28–37.
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): a review. J Sustain Metall. 2016;2:365–86.
Borra CR, Mermans J, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner Eng. 2016;92:151–9.
Borra CR, Pontikes Y, Binnemans K, Van Gerven T. Leaching of rare earths from bauxite residue (red mud). Miner Eng. 2015;76:20–7.
Boudreault R, Fournier J, Primeau D, Labrecque-Gilbert M-M. Processes for treating red mud. US Pat. US20150275330. 2015;26:895–9.
Dimas DD, Giannopoulou IP, Panias D. Utilization of alumina red mud for synthesis of inorganic polymeric materials. Miner Process Extr Metall Rev. 2009;30:211–39.
Jamieson E, Jones A, Cooling D, Stockton N. Magnetic separation of red sand to produce value. Miner Eng. 2006;19:1603–5.
Li Y, Chen H, Wang J, Xu F, Zhang W. Research on red mud treatment by a circulating superconducting magnetic separator. Environ Technol. 2014;35(10):1243–9.
Li Y, Wang J, Wang X, Wang B, Luan Z. Feasibility study of iron mineral separation from red mud by high gradient superconducting magnetic separation. Phys C Supercond. 2011;471:91–6.
Liu X, Zhang N. Utilization of red mud in cement production: a review. Waste Manag Res. 2011;29:1053–63.
Balomenos E, Panias D, Paspaliaris I. Energy and exergy analysis of the primary aluminum production processes: a review on current and future sustainability. Miner Process Extr Metall Rev. 2011;32:69–89.
Fursman OC, Mauser JE, Butler MO, Stickney WA. Utilization of red mud residues from alumina production. Report of Investigation 7454, US Bureau of Mines, Washington DC, 1970.
Wei D, Jun-Hui X, Yang P, Si-Yue S, Tao C. Iron extraction from red mud using roasting with sodium salt. Miner Process Extr Metall Rev. 2021;42:153–61.
UK Aluminium Industry Information Sheet. Aluminium and ecology. Birmingham: Aluminium Federation Ltd.; 2004.
Gostu S, Mishra B, Martins GP. Low temperature reduction of hematite in red-mud to magnetite. In: Ratvik A, editor. Light metals 2017. Cham: Springer; 2017. https://doi.org/10.1007/978-3-319-51541-0_10.
Lazou A, van der Eijk C, Balomenos E, Kolbeinsen L, Safarian J. On the direct reduction phenomena of bauxite ore using H2 gas in a fixed bed reactor. J Sustain Metall. 2020;6:227–38.
Kumar R, Srikanth S, Ramachamdrarao P. Phase stability in the Fe2O3–Al2O3–Na2CO3–C system and its implications in the processing of red mud. In: Leontiev LI, Kholkin AI, Belova VV, editors. Metall. Nonferrous Rare Met. Moscow: Russian Academy of Sciences; 2002. p. 137–53.
Mishra B, Staley A, Kirkpatrick D. Recovery of value-added products from red mud. Min, Metall Explorat. 2002;19:87–94.
Tian Y, Pan X, Yu H, Han Y, Tu G, Bi S. An improved lime sinter process to produce Al2O3 from low-grade Al-containing resources. In: Williams E, editor. Light Metals 2016. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-48251-4_1.
Tam PWY, Panias D, Vassiliadou V. Sintering optimisation and recovery of aluminum and sodium from greek bauxite residue. Miner. 2019;9:571.
Pei J, Pan X, Zhang Y, Yu H, Tu G. A novel process to fully utilize red mud based on low-calcium sintering. J Environ Chem Eng. 2021;9: 106754.
Lundquist RV, Blue DD. Role of alumina-to-silica mole ratio in the lime-soda sinter process. Report of Investigation 7434, US Bureau of Mines, Washington DC, 1970.
Liu W, Sun S, Zhang L, Jahanshahi S, Yang J. Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe. Miner Eng. 2012;39:213–8.
Kaussen F, Friedrich B. Soda sintering process for the mobilisation of aluminium and gallium in red mud. In: Pontikes Y, editor. Bauxite Residue Valoris. Best Pract. Conf. 5–7 October, 2015, Leuven. 2015. p. 157–64.
Guo YH, Gao JJ, Xu HJ, Zhao K, Shi XF. Nuggets production by direct reduction of high iron red mud. J Iron Steel Res Int. 2013;20:24–7.
Archambo MS, Kawatra SK. Utilization of bauxite residue: recovering iron values using the iron nugget process. Miner Process Extr Metall Rev. 2021;42(4):222–30.
Momber AW. The 50th anniversary of the death of Adolf Gustav Smekal (1895–1959), a pioneer in materials physics. J Mater Sci. 2010;45:750–8.
Baláž P, Achimovičová M, Baláž M, Billik P, Cherkezova-Zheleva Z, Criado JM, et al. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev. 2013;42:7571–637.
Baláž M. Environmental mechanochemistry. Berlin: Springer; 2021.
Baláž P. Mechanochemistry in nanoscience and minerals engineering. Berlin and Heidelberg: Springer; 2008. p. 1–413.
Alex TC, Kumar R. Surface and bulk activation of a siliceous bauxite during attrition milling. Int J Miner Process. 2017;160:32–8.
Alex TC, Kumar R, Roy SK, Mehrotra SP. Mechanical activation of Al-oxyhydroxide minerals – a review. Miner Process Extr Metall Rev. 2016;37(1):1–26.
Kumar R, Alex TC, Jha MK, Khan ZH, Mahaptra SP, Mishra CR. Mechanochemistry and the Bayer process of alumina production. In: Tabereaux AT, editor. Light Met. 2004. TMS (The Minerals, Metals & Materials Society); 2004. p. 31–4.
Kumar R, Alex TC, Khan ZH, Mahapatra SP, Mehrotra SP. Mechanical activation of bauxite potential and prospects in the bayer process. In: Kvande H, editor. Light Met. 2005. TMS (The Minerals, Metals & Materials Society); 2005. p. 77–9.
Picaro T. Red mud processing [Internet]. Pat. Appl. WO/1997/029992. 1997. p. 1–18. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1997029992
Eriksson G, Hack K. ChemSage—a computer program for the calculation of complex chemical equilibria. Metall Trans B. 1990;21:1013–23.
The theoretical background of FactSage - CRCT - [PDF Document] [Internet]. [cited 2022 May 11]. Available from: https://documents.pub/document/the-theoretical-background-of-factsage-crct.html?page=1
Smith WR, Missen RW. Chemical reaction equilibrium analysis: theory and algorithms. New York: Wiley; 1982.
Mendham J, Denney RC, Barnes JD, Thomas M, Sivasankar B. Vogel’s quantitative chemical analysis. 6th ed. London: Pearson Publisher; 2009.
Ghosh A, Chatterjee A. Ironmaking and steelmaking: theory and practice Ist. New Delhi: Prentice-Hall of India Pvt. Ltd.; 2008.
Juhasz AZ, Opoczky L. Mechanical activation of minerals by grinding: pulverizing and morphology of particles. New York: Ellis Horwood Limited; 1994.
Meher SN. Thermal analysis of Nalco red mud. Int J Chem Stud. 2014;1:1–9.
Castaldi P, Silvetti M, Santona L, Enzo S, Melis P. XRD, FTIR, and thermal analysis of bauxite ore-processing waste (red mud) exchanged with heavy metals. Clays Clay Miner. 2008;56:461–9.
Antunes MLP, Couperthwaite SJ, Da Conceição FT, De Jesus CPC, Kiyohara PK, Coelho ACV, et al. Red mud from Brazil: thermal behavior and physical properties. Ind Eng Chem Res. 2011;51:775–9.
Atasoy A. The comparison of the Bayer process wastes on the base of chemical and physical properties. J Therm Anal Calorim. 2007;90:153–8.
Earnest CM, Gann K, Stong B. Improved quantification of gibbsite in bauxite ores by thermogravimetric methods (TGA and DTG). Adv Appl Chem Biochem. 2018;1:9–17.
Shivendra S, Abhilash, Meshram P, Pandey BD 2016 Metallurgical processes for the recovery and recycling of lanthanum from various resources - A review. Hydrometallurgy, 160:47–59.
Yusiharni E, Gilkes R. Rehydration of heated gibbsite, kaolinite and goethite: an assessment of properties and environmental significance. Appl Clay Sci. 2012;64:61–74.
Gan BK, Taylor Z, Xu B, Van Riessen A, Hart RD, Wang X, et al. Quantitative phase analysis of bauxites and their dissolution products. Int J Miner Process. 2013;123:64–72.
Zhang TA, Wang Y, Lu G, Liu Y, Zhang W, Zhao Q. Comprehensive utilization of red mud: Current research status and a possible way forward for non-hazardous treatment. In: Martin, O. (eds) Light Metals 2018. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72284-9_18
Wilson SJ. The dehydration of boehmite, γ-AlOOH, to γ-Al2O3. J Solid State Chem. 1979;30:247–55.
Sanders SR. Determination of alumina and iron phases in bauxite by DTG analysis. Light Met. 1982. Warrendale: TMS ( The Minerals , Metals & Materials Society ); 1982. p. 29–43.
Sglavo VM, Campostrini R, Maurina S, Carturan G, Monagheddu M, Budroni G, et al. Bauxite red mud in the ceramic industry. Part 1: thermal behaviour. J Eur Ceram Soc. 2000;20:235–44.
Alex TC, Kumar R, Roy SK, Mehrotra SP. Anomalous reduction in surface area during mechanical activation of boehmite synthesized by thermal decomposition of gibbsite. Powder Technol. 2011;208:128–36.
Alex TC, Kumar R, Roy SK, Mehrotra SP. Mechanically induced reactivity of gibbsite: part 1 planetary milling. Powder Technol. 2014;264:105–13.
Singla R, Alex TC, Kumar R. On mechanical activation of glauconite: physicochemical changes, alterations in cation exchange capacity and mechanisms. Powder Technol. 2020;360:337–51.
Liu X, Han Y, He F, Gao P, Yuan S. Characteristic, hazard and iron recovery technology of red mud—a critical review. J Hazard Mater. 2021;420: 126452.
Raspopov NA, Korneev VP, Averin VV, Lainer YA, Zinoveev DV, Dyubanov VG. Reduction of iron oxides during the pyrometallurgical processing of red mud. Russ Metall. 2013;2013:33–7.
Li G, Liu M, Rao M, Jiang T, Zhuang J, Zhang Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J Hazard Mater. 2014;280:774–80.
Li X, Xiao W, Liu W, Liu G, Peng Z, Zhou Q, et al. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans Nonferrous Met Soc China. 2009;19:1342–7.
Zhou Q, Li C, Li X, Peng Z, Liu G, Qi T. Reaction behavior of ferric oxide in system Fe2O3–SiO2–Al2O3 during reductive sintering process. Trans Nonferrous Met Soc China. 2016;26:842–8.
FToxid - Oxide Phase Diagrams [Internet]. 2016 [cited 2022 Jun 27]. Available from: https://www.crct.polymtl.ca/fact/documentation/FToxid/FToxid_Figs.htm
Zhou G, Wang Y, Qi T, Zhou Q, Liu G, Peng Z, et al. Comprehensive utilization of Al-goethite-containing red mud treated through low-temperature sodium salt-assisted roasting-water leaching. J Sustain Metall. 2022;8:825–36.
Siriwardane RV, Poston JA, Robinson C, Simonyi T. Effect of additives on decomposition of sodium carbonate: precombustion CO2 capture sorbent regeneration. Energy Fuels. 2011;25:1284–93.
Vance K, Falzone G, Pignatelli I, Bauchy M, Balonis M, Sant G. Direct carbonation of Ca(OH)2 using liquid and supercritical CO2: implications for carbon-neutral cementation. Ind Eng Chem Res. 2015;54:8908–18.
Hayes PC. Process principles in minerals and materials production – with a focus on metal production and recycling. 4th ed. Brisbane, Queensland, Australia: Hayes Publishing Co.; 2021.
Seth BL, Ross HU. The effect of lime on the reducibility of iron-oxide agglomerates. Can Metall Q. 1963;2(1):15–30.
Ueda S, Watanabe K, Inoue R, Ariyama T. Catalytic effect of Fe, CaO and molten oxide on the gasification reaction of coke and biomass char. ISIJ Int. 2011;51:1262–8.
Acknowledgements
The red mud used in the study was received from National Aluminium Company (India). The authors sincerely acknowledge characterisation support received from Dr. S.K. Das (SEM-EDS), Dr. B Ravikumar (XRD), Dr. D. Mishra (analytical) and Dr. N. Randhawa (thermal analysis) (all from CSIR-NML). The authors would like to sincerely thank Prof. Rajiv Shekhar (Director, IIT(ISM) and Dr. Indranil Chattoraj (Director, CSIR-NML) for their encouragement and support for this work. The first author would also like to express his gratitude to Prof. Seshadri Seetharaman (Royal Institute of Technology, Stockholm, Sweden) for useful discussion and providing valuable ideas during his visit to IIT(ISM) in early 2020.
Funding
The authors, individually or collectively, did not receive any funding to purse the work reported in this paper.
Author information
Authors and Affiliations
Contributions
Conceptualization contributed by RK; methodology and experimental work contributed by TCA; formal analysis and investigation contributed by RK, TCA; writing—original draft preparation contributed by TCA; writing—review and editing contributed by RK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Alex, T.C. Phase stability and role of mechanical activation in the chemical beneficiation of red mud. J Therm Anal Calorim 148, 9813–9827 (2023). https://doi.org/10.1007/s10973-023-12350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12350-7