Abstract
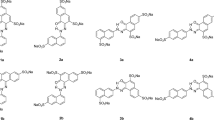
Three ethers with chlorine and two azo groups of anthracene, which are synthetic dyes, were prepared. The compounds were named B2ClA, B4ClA and BDClA. For these yellow compounds, called bis-azobisethers, the thermal, optical, spectral, adhesion and biological properties were investigated. The compounds are thermally stable up to about 240 °C, at higher temperatures producing, in the air, the complete oxidative decomposition, in two strongly exothermic stages, up to temperatures close to 600 °C. FTIR spectroscopy identified the components of the studied molecules, and UV-Vis spectroscopy determined the quantum efficiency of bis-azo compounds. The laser electronic fluorescence established the electronic levels between which the transitions occur when the component atoms of the molecules are excited by an Ar+ laser, as well as the energies of the quanta that accompany these transitions. Bis-azo chlorinated compounds of anthracene have optical anisotropy, proving the birefringence of their crystals. The studied compounds adhere to the glass surface, forming submicron crystallites, with the roughness of 80–100 nm. Bis-azo chlorinated compounds of anthracene interact poorly with proteins and do not show significant antioxidant properties.


















Similar content being viewed by others
References
Rotaru A, Moanță A. Azoic dyes: from thermal properties to a wide range of applications. In: Chapter in: Advanced Engineering Materials. Recent Developments for Medical, Technological and Industrial Applications, Academica Greifswald, 978–3–940237–38–5, Germany, 2016.
Constantinescu C, Morîntale E, Ion V, Moldovan A, Luculescu C, Dinescu M, Rotaru P. Thermal, morphological and optical investigations of Cu(DAB)2 thin films produced by matrix-assisted pulsed laser evaporation and laser-induced forward transfer for sensor development. Thin Solid Films. 2012;520:3904–9.
Rotaru A, Moanță A, Sălăgeanu I, Budrugeac P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers Part I. Decomposition of 4-[(4-chlorobenzyl)oxy]-4’-nitro-azobenzene. J Therm Anal Calorim. 2007;87:395–400.
Rotaru A, Kropidłowska A, Moanță A, Rotaru P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers Part II. Non-isothermal study of three liquid crystals in dynamic air atmosphere. J Therm Anal Calorim. 2008;92:233–8.
Rotaru A, Moanță A, Rotaru P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers Part III. Non-isothermal study of 4-[(4-chlorobenzyl)oxy]-4’-chloroazobenzene in dynamic air atmosphere. J Therm Anal Calorim. 2009;95:161–6.
Rotaru A, Moanță A, Popa G, Rotaru P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers Part IV. Non-isothermal kinetics of 2-allyl-4-((4-(4-methylbenzyloxy)phenyl) diazenyl)phenol in air flow. J Therm Anal Calorim. 2009;97:485–91.
Rotaru A, Constantinescu C, Rotaru P, Moanță A, Dumitru M, Socaciu M, Dinescu M, Segal E. Thermal analysis and thin films deposition by matrix assisted pulsed laser evaporation of a 4CN type azomonoether. J Therm Anal Calorim. 2008;92:279–84.
Al Reza S, Hasan M, Kamruzzaman, Hossain I, Zubair A, Bari L, Abedin Z, Reza A, Khalid-Bin-Ferdaus K, Faisal Haque K, Islam K, Ahmed MU, Hossain K. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci Nutr. 2019;7:667–77.
Yamjala K, Nainar MS, Ramisetti NR. Methods for the analysis of azo dyes employed in food industry – A review. Food Chem. 2016;192:813–24.
Leulescu M, Rotaru A, Pălărie I, Moanță A, Cioateră N, Popescu M, Morîntale E, Bubulică MV, Florian G, Hărăbor A, Rotaru P. Tartrazine: physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. J Therm Anal Calorim. 2018;134:209–31.
Leulescu M, Pălărie I, Moanță A, Cioateră N, Popescu M, Morîntale E, Văruț MC, Rotaru P. Brown HT Physical, thermal and biophysical properties of the food azo dye. J Therm Anal Calorim. 2019;136:1249–68.
Leulescu M, Iacobescu G, Bojan M, Rotaru P. Ponceau 4R azoic red dye Thermal behavior, optical anisotropy and terahertz spectroscopy study. J Therm Anal Calorim. 2019;138:2091–101.
Kostić V, Stafilov T, Stojanoski K. HPLC investigation of the degradation of some artificial azo food colorants in the presence of ascorbic acid. Contributions, Sec Math Tech Sci. 2008;29:89–98.
Selvam K, Swaminathan K, Chae KS. Decolourization of azo dyes and a dye industry effluent by a white rot fungus Thelephora sp. Biores Technol. 2003;88:115–9.
Saratale RG, Saratale GD, Kalyani DC, Chang JS, Govindwar SP. Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR. Biores Technol. 2009;100:2493–500.
Pinheiro HM, Touraud E, Thomas O. Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigments. 2004;61:121–39.
Máximo C, Pessoa Amorim MT, Costa-Ferreira M. Biotransformation of industrial reactive azo dyes by Geotrichum sp. CCMI 1019. Enzyme and Microbial Technology 2003;32:145–51.
Pandey A, Singh P, Iyengar L. Bacterial decolorization and degradation of azo dyes. Int Biodet Biodegrad. 2007;59:73–84.
Meehan C, Bjourson AJ, McMullan G. Paenibacillus azoreducens sp. nov., a synthetic azo dye decolorizing bacterium from industrial wastewater. Int Journal System Evolut Microbiol. 2001;51:1681–5.
Rodriguez CS. Decolouration of industrial azo dyes by crude laccase from Trametes hirsute. J Hazard Mat. 2007;148:768–70.
Ahmad F, Lee JW, Jeon YJ, Jamil M. Enhanced electro-optical characteristics of dispersedAzo dye doped polymer-liquid crystal display. Optoelectron Adv Mat - Rapid Commun. 2017;11:603–7.
Turgay O, Ersoz G, Atalay S, Forss J, Welander U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Separation Purification Technology. 2011;79:26–33.
Mohan SV, Rao NC, Prasad KK, Karthikeyan J. Treatment of simulated Reactive Yellow 22 (Azo) dye effluents using Spirogyra species. Waste Manage. 2002;22:575–82.
Huang CY, Lin YR, Lo KY, Lee CR. Dynamics of photoalignment in azo-dye-doped liquid crystals. Appl Phys Let. 2008;93: 181114.
Fuh AYG, Chen JC, Huang SY, Cheng KT. Binary liquid crystal alignments based on photoalignment in azo dye-doped liquid crystals and their application. Appl Phys Let. 2010;96: 051103.
Moanță A, Ionescu C, Rotaru P, Socaciu M, Hărăbor A. Structural characterization, thermal investigation, and liquid crystalline behavior of 4-[(4-chlorobenzyl)oxy]-3,40-dichloroazobenzene. J Therm Anal Calorim. 2010;102:1079–86.
Rotaru A, Dumitru M. Thermal behaviour of CODA azoic dye liquid crystal and nanostructuring by drop cast and spin coating techniques. J Therm Anal Calorim. 2017;127:21–32.
Rotaru A, Moanță A, Constantinescu C, Dumitru M, Manolea HO, Andrei A, Dinescu M. Thermokinetic study of CODA azoic liquid crystal and thin films deposition by matrix-assisted pulsed laser evaporation. J Therm Anal Calorim. 2017;128:89–105.
Shuji I, Akio M, Mikio S, Masaharu K, Tomio Y, Junko I. Liquid crystal composition containing azo dyes. EUROPEAN PATENT 0 087 248 A1, 1983.
Dixit S, Vora RA. Study of novel liquid crystalline materials with lateral ester group. Int J Eng Res Technol. 2015;4:651–5.
Al-Hamdani UJ, Tarik E. Gassim TE, Radhy HH. Synthesis and characterization of azo compounds and study of the effect of substituents on their liquid crystalline behavior. Molecules. 2010;15:5620–8.
Yue Y, Norikane Y, Azumi R, Koyama E. Light-induced mechanical response in crosslinkedliquid-crystalline polymers with photoswitchable glass transition temperatures. Nat Commun. 2018;9:3234.
Zhao HW, He XZ, Zheng JJ, Song WL, Meng FB, Hu JS. Synthesis and properties of chiral azo-liquid crystalline terpolymer containing cyano mesogenic units. Liq Cryst. 2017;44:2379–90.
Onga LK, Hab ST, Yeapc GY, Lin HC. Heterocyclic pyridine-based liquid crystals: synthesis and mesomorphic properties. Liq Cryst. 2018;45:1574–84.
Konstantinov I. Liquid crystalline properties of some monomeric azo- and azoxybenzenes and their polymers. Journal de Physique Colloques. 1979;40:475–7.
Zhai BG, Chen LL, Huang YM. Photochemistry in an azo-containing banana-shaped liquid crystal. Key Eng Mat. 2010;428–429:202–5.
Ouskova E, Vapaavuori J, Kaivola M. Self-orienting liquid crystal doped with polymer-azo-dye complex. Opt Mat Express. 2011;1:1463–70.
Jull EIL, Gleeson HF. All-optical responsive azo-doped liquid crystal laser protection filter. Opt Express. 2018;26:34179–84.
Inglot K, Martynski T, Bauman D. Molecular organization and aggregation in Langmuir and Langmuir-Blodgett films of azo dye/liquid crystal mixtures. Opto−Electronics Rev. 2009;17:120–8.
Becchi M, Janossy I, Shankar Rao DS, Statman D. Anomalous intensity dependence of optical reorientation in azo-dye-doped nematic liquid crystals. Phys Rev E. 2004;69: 051707.
Oh SW, Baek JM, Kim SH, Yoon TH. Optical and electrical switching of cholesteric liquid crystals containing azo dye. RSC Adv. 2017;7:19497–501.
Li H, Wang J, Wang C, Zeng P, Pan Y, Yang Y. Enhanced diffraction properties of photoinduced gratings in nematic liquid crystals doped with disperse red 1. Proc Jpn Acad., Ser B. 2016;92:330–5.
Epure EL, Lisa G, Simion G, Simion A, Ciobanu CI, Carlescu I. Thermal behavior, decomposition mechanism by TG/MS/FTIR technique and theoretical study of some symmetric and asymmetric bent-core liquid crystals based on 2,7-dihydroxynaphthalene. J Therm Anal Calorim. 2022;147:12033–45.
Husain A, Sawaya W, Al-Omair A, Al-Zenki S, Al-Amiri H, Ahmed N, Al-Sinan M. Estimates of dietary exposure of children to artificial food colours in Kuwait. Food Additives Contaminants. 2006;23:245–51.
Cheng Q, Xia S, Tong J, Wu K. Highly-sensitive electrochemical sensing platforms for food colourants based on the property-tuning of porous carbon. Anal Chim Acta. 2015;887:75–81.
Zhang Y, Hu L, Liu X, Liu B, Wu K. Highly-sensitive and rapid detection of ponceau 4R and tartrazine in drinks using alumina microfibers-based electrochemical sensor. Food Chem. 2015;166:352–7.
Dubin P, Wright KL. Reduction of azo food dyes in cultures of Proteus vulgaris. Xenobiotica. 1975;5:563–71.
Uysal H, Genc S, Ayar A. Toxic e_ects of chronic feeding with food azo dyes on Drosophila melanogaster Oregon R. Scientia Iranica C. 2017;24:3081–6.
Sen SK, Raut S, Bandyopadhyay P, Raut S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol Rev. 2016;30:112–33.
Sudha M, Saranya A, Selvakumar G, Sivakumar N. Microbial degradation of azo dyes: A review. Int J Curr Microbiol App Sci. 2014;3:670–90.
Lade HS, Waghmode TR, Kadam AA, Govindwar SP. Enhanced biodegradation and detoxification of disperse azo dye Rubine GFL and textile industry effluent by defined fungal-bacterial consortium. Int Biodeterioration Biodegradation. 2012;72:94–107.
Mielgo I, Moreira MT, Feijoo G, Lema JM. A packed-bed fungal bioreactor for the continuous decolourisation of azo-dyes (Orange II). J Biotechnology. 2001;89:99–106.
Raschig M, Ramirez-Zavala B, Wiest J, Saedtler M, Gutmann M, Holzgrabe U, Morschausser J, Meinel L. Azobenzene derivatives with activity against drug-resistant Candida albicans and Candida auris. Arch Pharm. 2022. https://doi.org/10.1002/ardp.202200463.
Leo L, Loong C, Ho XL, Raman MFB, Yue M, Suan T, Loke WM. Occurrence of azo food dyes and their effects on cellular inflammatory responses. Nutrition. 2018;46:36–40.
Gholami-Borujeni F, Mahvi AH, Naseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M. Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Res J Chem Environ. 2011;15:217–22.
Fnfoon DY, Al-Adilee KJ. Synthesis and spectral characterization of some metal complexes with new heterocyclic azo imidazole dye ligand and study biological activity as anticancer. J Mol Struc. 2023;1271, DOI:https://doi.org/10.1016/j.molstruc.2022.134089.
Kamali S, Orojloo M, Arabahmadi R, Amani S. Design and synthesis of a novel azo-Schiff base ligand: Its application as a colorimetric chemosensor for selective detection of Ni2+ and CN− in aqueous-organic media, computational studies, antimicrobial properties, and molecular logic circuits. J Photochem Photobiology A-Chem. 2022;433, DOI:https://doi.org/10.1016/j.jphotochem.2022.114136.
Mohammed KF, Hasan HA. Synthesis, chemical and biological activity Studies of azo-Schiff base ligand and its metal complexes. Chem Methodol. 2022;12:905–13.
Abdelaziz A, Gaber M, El-Wakiel N, El-Sayed YS. Ag(I), In (III), and Sn (II) chelates of azo mesalamine drug: Characterization, DFT studies, molecular docking and biological evaluation. Appl Organomet Chem. 2022. https://doi.org/10.1002/aoc.6944.
Monajjemi M, Kandemirli F, Mollaamin F. Azo-dye antibacterial with nanotube-[SiO2(OH)(2)] system for drug delivery. Biointerface Res Appl Chem. 2022;12:8515–26.
Kumar M, Khushi K, Bhardwaj A, Deb DK, Singh N, Elahi D, Sharma S, Bajpai G, Srivastava A. In-vitro study for Ibuprofen encapsulation, controlled release and cytotoxicity improvement using excipient-drugs mixed micelle. Colloids Surf A Phycochem Eng Aspects. 2022;654, Doi:https://doi.org/10.1016/j.colsurfa.2022.130057.
Moanță A, Ionescu C, Drăgoi M, Tutunaru B, Rotaru P. A new azo-ester: 4-(phenyldiazenyl)phenyl benzene sulfonate—spectral, thermal, and electrochemical behavior and its antimicrobial activity. J Therm Anal Calorim. 2015;120:1151–61.
Kazem-Rostami M. Factors influencing the thermal stability of azo and bisazo compounds. J Therm Anal Calorim. 2020;140:613–23.
Shikhaliyev NQ, Kuznetsov ML, Maharramov AM, Gurbanov AV, Ahmadova NE, Nenajdenko VG, Mahmudov KT, Pombeiro AJL. Noncovalent interactions in the design of bis-azo dyes. Cryst Eng Comm. 2019;21:5032–8.
Janssens S, Breukers R, Swanson A, Raymond S. Photoinduced properties of Bis-azo chromophore host guest systems-birefringence and all optical tuneable polymer waveguide Bragg gratings. J Appl Phys. 2017;122: 023107.
Garcia-Amorós J, Reig M, Cuadrado A, Ortega M, Nonell S, Velasco D. A photoswitchable bis-azo derivative with a high temporal resolution. Chem Commun. 2014;50:11462–4.
Nourmohammadiana F, Alikhanic MY, Gholamia MD, Abdi AA. Benzothiazole-based bis-azo cationic fluorescent dyes with extended conjugated systems: synthesis and properties. J Appl Solution Chem Modeling. 2015;4:83–94.
Patel DR, Patel NS, Patel HS, Patel KC. Synthesis, characterization and application of novel bisazo reactive dyes on various fibers. Orbital Elec J Chem. 2011;3:57–67.
Scutaru D, Carlescu I, Bulai ER, Ciobanu CI, Lisa G, Hurduc N. Bent-Core liquid crystals: Structures and mesomorphic properties. In Liquid Crystals - Self-organized soft functional materials for advanced applications, IntechOpen House, Iași, 2018.
Chigrinov V, Prudnikova E, Kozenkov V, Kwok H, Akiyama H, Kawara T, Takada H, Takatsu H. Synthesis and properties of azo dye aligning layers for liquid crystal cells. Liq Cryst. 2002;29:1321–7.
Saad GR, Ahmed NHS, Abdelgawad A, Fahmi AA, Kaddah MM, Magdi M. Naoum MM. Influence of lateral methyl and terminal substituents on the mesophase behaviour of four rings azo-ester liquid crystal compounds. Liquid Crystals. 2019;46:1285–97.
Paebumrung P, Petsom A, Thamyongkit P. Cardanol-based bis(azo) dyes as a gasoline 91 colorant. J Am Oil Chem Soc. 2012;89:321–8.
Mahmoud WH, Omar MM, Sayed FN. Synthesis, spectral characterization, thermal, anticancer and antimicrobial studies of bidentate azo dye metal complexes. J Therm Anal Calorim. 2016;124:1071–89.
Badea M, Emandi A, Marinescu D, Elena Cristurean E, Olar R, Braileanu A, Budrugeac P, Segal E. Thermal stability of some azo-derivatives and their complexes 1-(2-benzothiazolyl)-3-methyl-4-azo-pyrazil-5-one derivatives and their Cu(II) complexes. J Therm Anal Calorim. 2003;72:525–31.
Leulescu M, Rotaru A, Moanță A, Iacobescu G, Pălărie I, Cioateră N, Popescu M, Criveanu MC, Morîntale E, Bojan M, Rotaru P. Azorubine: Physical, thermal and bioactive properties of the widely employed food, pharmaceutical and cosmetic red azo-dye material. J Therm Anal Calorim. 2021;143:3945–67.
Radu S, Iovu M. Éthers-oxydes polymères des composés azoïques. Hétéropolycondensation du azodiphénolate-4,40 de sodium avec des dérivés bis(chlorométhylés) du benzène. Makromol Chem. 1975;176:883–90.
Radu S, Jianu A, Rau G. Synthese et analyse spectrale des α, α’-bis-(azobenzene-4-oxy)-9,10-dimethyleantracenes. Ann Univ Craiova Chim Ser. 1998;27:38–42.
Radu S, Rau G, Moanță A. Bis[(Phenylazo)biphenylenoxymethylen] anthracenes. Ann Univ Craiova Chim Ser. 2000;29:164–9.
Carabet CA, Moanță A, Palarie I, Iacobescu G, Rotaru A, Leulescu M, Popescu M, Rotaru P. Physical, thermal and biological properties of yellow dyes with two azodiphenylether groups of anthracene. Molecules. 2020;25:5757.
https://www.microscopyu.com/techniques/polarized-light/principles-of-birefringence
Pogany I, Banciu M. Physical methods in organic chemistry. Bucharest: Scientific Publishing House; 1972.
Pălărie I. Optics of anisotropic materials. Craiova: Universitaria Publishing House; 2010.
Shortley G, Williams D. Elements of Physics. Englewood Cliffs, New Jersey: Prentice-Hall, Inc.; 1971.
Podstawka E, Swiatłowska M, Borowiec E, Proniewicz LM. Food additives characterization by infrared, Raman, and surface-enhanced Raman spectroscopies. J Raman Spectrosc. 2007;38:356–63.
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR. Introduction to spectroscopy. Brooks/Cole Cengage Learning, Belmont, USA, 2009.
Peica N. Vibrational spectroscopy and density functional theory calculations on biological molecules. Dissertation. Würzburg; 2006.
Socrates G. Infrared and Raman characteristic group frequencies: tables and charts. 3rd ed. Chichester: Wiley; 2004.
Silverstein RM, Webster FX, Kiemle DJ. Spectrometric identification of organic compounds. New York: John Wiley & sons, Inc.; 2005.
Gunasekaran S, Sailatha E, Seshadri S, Kumaresan S. FTIR FT Raman spectra and molecular structural confirmation of isoniazid. Indian J Pure Appl Phys. 2009;47:12–8.
Lin-Vien D, Colthup NB, Fateley WB, Graselli JG. The handbook of infrared and raman characteristic frequencies of organic molecules. Boston: Academic Press; 1991.
Moanță A, Samide A, Rotaru P, Ionescu C, Tutunaru B. Synthesis and characterization of novel furoate azodye using spectral and thermal methods of analysis. J Therm Anal Calorim. 2015;119:1039–45.
Beral E, Zapan M. Organic Chemistry. Bucharest: Technical Publishing House; 1973.
Pălărie I. Spectroscopy. Craiova: Practical works. Universitaria Publishing House; 2004.
Pălărie I, Văruț MC, Chirigiu LME. Method of Determination of Rivanol by Laser Induced Fluoroscence. Rev Chim Bucharest. 2019;70:140–2.
Leulescu M. Physical aspects of the behavior of biological active materials. PhD thesis, University of Craiova, 2019.
Jianu D, Soare B, Matei L. Microscopic optical properties of transparent minerals in polarized light. www.old.unibuc.ro. 2007.
https://www.microscopyu.com/techniques/polarized-light/principles-of-birefringence.
Iacobescu G, Moţoc C. Liquid crystals. Craiova: Physical properties and applications. Universitaria Publishing House; 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moanță, A., Carabet, A.C., Pălărie, I. et al. Thermal, physical and biological properties of new etheric dyes with chlorine and two azo groups of anthracene. J Therm Anal Calorim 148, 4615–4639 (2023). https://doi.org/10.1007/s10973-023-12016-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12016-4