Abstract
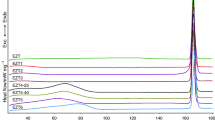
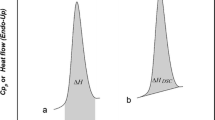
The check of the solid phase in which the active principles crystallize is an activity of primary importance, both in the preformulation studies and in the quality control of pharmaceutical products. Indeed, it is necessary to ensure that only the polymorph of interest is present in the dosage form, as well as in the various stages leading to its preparation. The regulatory agencies underline the importance of limiting the presence, in the pharmaceutical solids, of polymorphic impurities which, having different physico-chemical, technological and pharmaceutical properties, could compromise the efficacy, the stability and the safety of the product. The present work fits into this problem and is configured as a response to the industrial need to identify the presence of an unwanted polymorph in batches of powder of an active ingredient, also in systems in which these determinations show serious problems. In particular, the studies here performed respond to the need to develop a method that allows to detect and quantify the presence of polymorphic impurity in dexketoprofen trometamol samples by the DSC technique. The main critical points of this system are constituted by the fact that the melting enthalpies of the two polymorphs (A and B) are very similar and the difference between the onset temperatures of their melting is just 2 °C. This second point makes it impossible to obtain an adequate resolution of the melting peaks, even when the experimental conditions are appropriately selected. A system characterized by this thermal behavior, in the scientific literature, is considered "impossible". The success of the method developed in this work is to be attributed, on the one side, to the methods adopted in the preparation of the physical mixtures of the two polymorphs, which need to have the composition as homogeneous as possible, and on the other side, to the idea of performing a conditioning of the samples that makes the low-melting temperature polymorph thermally inert. The polymorphic mixtures treated in this way show only the fusion of the high-melting temperature polymorph whose enthalpy decreases linearly with the increase of impurity present in the mixture. The method proposed here allows to detect the polymorphic impurity even when it is present in a very low percentage, equal to 0.3, while it is possible to quantify it reliably for contents higher than 2%.











Similar content being viewed by others
References
International Conference on Harmonization Q6A Guideline. Specifications for New Drug Substances and Products: Chemical Substances, 1999 October.
Raw AS, Furness MS, Gill DS, Adams RC, Holcombe FO Jr, Yu LX. Regulatory considerations of pharmaceutical solid polymorphism in abbreviated new drug applications (ANDAs). Adv Drug Deliv Rev. 2004;56:397–414.
Correa JCR, Perissinato AG, dos Reis Serra CH, Trevisan MG, Salgado HRN. Polymorphic stability of darunavir and its formulation. J Therm Anal Calorim. 2016;123:2185–90.
Dias SBT, Nascimento TG, Santos AFO, Viana IMMN, Almeida RM, Basilio Junior ID, Macedo RO, de Araujo-Junior JX. Polymorphic characterization and compatibility study of clozapine: implications on its stability and some biopharmaceutics properties. J Therm Anal Calorim. 2015;120:795–805.
Brittain HG. Effects of mechanical processing on phase composition. J Pharm Sci. 2002;91:1573–80.
Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Deliv Rev. 2004;56:335–47.
Tiwary AK. Modification of crystal habit and its role in dosage form performance. Drug Dev Ind Pharm. 2001;27:699–709.
Airaksinen S, Karjalainen M, Räsänen E, Rantanen J, Yliruusi J. Comparison of the effects of two drying methods on polymorphism of theophylline. Int J Pharm. 2004;276:129–41.
Bruni G, Berbenni V, Milanese C, Girella A, Cardini A, Lanfranconi S, Marini A. Determination of the nateglinide polymorphic purity through DSC. J Pharm Biomed Anal. 2011;54:1196–9.
McGregor C, Saunders MH, Buckton G, Saklatvala RD. The use of high-speed differential scanning calorimetry (Hyper-DSC) to study the thermal properties of carbamazepine polymorphs. Thermochim Acta. 2004;417:231–7.
Shah B, Kakumanu VK, Bansal AK. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J Pharm Sci. 2006;95:1641–65.
Vitez I. Utilization of DSC for pharmaceutical crystal form quantitation. J Therm Anal Cal. 2004;78:33–45.
Hanna M, Moon YJ. A review of dexketoprofen trometamol in acute pain. Curr Med Res Opin. 2019;35:189–202.
Carganico G, Mauleón D, García ML. A novel arylpropionic derivative, its method of preparation and its application as an analgesic. International Patent WO-94/11332. 1992.
Esparza-Villalpando V, Pozos-Guillen A, Masuoka-Ito D, Gaitan-Fonseca C, Chavarria-Bolanos D. Analgesic efficacy of preoperative dexketoprofen trometamol: a systematic review and meta-analysis. Drug Dev Res. 2018;79:47–57.
Bosch M, Mannucci S, Torras E, Falorni R, Gonzales JM. Polymorphic forms of dexketoprofen trometamol, preparation and pharmaceutical compositions thereof. European Patent EP1739072 A1. 2007.
Farshi F, Soylemez S, Koc , Durmus S. A process for preparing dexketoprofen trometamol form A and form B crystals. International Patent WO2011/001213 A1. 2009.
Rossi P, Paoli P, Chelazzi L, Milazzo S, Biagi D, Valleri M, Ienco A, Valtancoli B, Conti L. Relationships between anhydrous and solvated species of dexketoprofen trometamol: a solid-state point of view. Cryst Growth Des. 2020;20:226–36.
Author information
Authors and Affiliations
Contributions
AC contributed to conceptualization; GB provided methodology; GB and DC performed formal analysis and investigation; GB performed writing—original draft preparation; GB and DC performed writing—review and editing; GB and AC provided resources; DC performed visualization; CM carried out data curation; GB performed supervision.
Corresponding author
Ethics declarations
Ethical approval
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruni, G., Capsoni, D., Milanese, C. et al. Polymorphic quantification of dexketoprofen trometamol by differential scanning calorimetry. J Therm Anal Calorim 148, 1949–1958 (2023). https://doi.org/10.1007/s10973-022-11870-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11870-y