Abstract
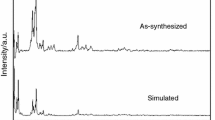
MIL-53(Cu), belonging to the group of metal–organic frameworks (MOFs) with terephthalate as a ligand, was prepared. The success of the synthesis was confirmed by XRD, FTIR, SEM and chemical analysis. The MOF thermal behavior was studied by thermogravimetry, in situ and ex situ XRD and mass spectrometry, using different atmospheres (inert, oxidizing and reducing). This MOF had a higher stability when the inert atmosphere was used (622 K), and this was lower (559 K) under an oxidizing atmosphere. The integrated intensities of the main peak of each phase in the X-ray diffractograms showed that under inert or reducing atmospheres (CO or H2) the decomposition generated metallic copper. Under oxidizing atmosphere, a mixture of copper oxides (Cu2O and CuO) was obtained, in which Cu2O was an intermediate, with only traces observed at 773 K. The FWHM calculation at the main peak of each phase indicated the formation of larger crystallites under hydrogen atmosphere and smaller ones under CO. There was a tendency of the formed copper oxides to sinter in the presence of air. The main volatile compounds formed during the thermal treatment of the MOF (decomposition of terephthalate) were CO2 and benzene. Thus, this study contributes to the development of new copper-based catalysts by the thermal treatment of MOF, which allows greater control of the phases formed (metallic Cu or CuO).

















Similar content being viewed by others
References
Furukawa H., Cordova KE., O’Keeffe M., Yaghi OM. The chemistry and applications of metal-organic frameworks. Science. 2013;341:123044-1-12. https://doi.org/10.1126/science.1230444.
Healy C., Patil KM, Wilson BH, Hermanspahn L, Harvey-Reid NC, Howard BI, Kleinjan C, Kolien J, Payet F, Telfer SG, Kruger PE, Bennett TD. The thermal stability of metal-organic frameworks. Coord. Chem. 2020;419:213388-1-20. https://doi.org/10.1016/j.ccr.2020.213388.
Khan S, Guan Q, Liu Q, Qin Z, Rasheed B, Liang X, Yang X. Synthesis, modifications and applications of MILs metal-organic frameworks for environmental remediation: The cutting-edge review. Sci. Total Environ. 2021;810:152279-1–33. https://doi.org/10.1016/j.scitotenv.2021.152279.
Feijani EA, Mahdavi H, Tavasoli A. Poly(vinylidene fluoride) based mixed matrix membranes comprising metal organic frameworks for gas separation applications. Chem Eng Res Des. 2015;96:87–102. https://doi.org/10.1016/j.cherd.2015.02.009.
Silva Junior OJ, Monteiro AFF, Oliveira JBL, Araújo AMU, Silva DG, Kulesza J, Barros BS. Coordination polymer-derived CuO catalysts for oxidative degradation of methylene blue. Mater. Chem. Phys. 2019;235:121737-1–8. https://doi.org/10.1016/j.matchemphys.2019.121737.
Li B, Zheng JQ, Guo JZ, Dai CQ, A novel route to synthesize MOFs-derived mesoporous dawsonite and application in elimination of Cu(II) from wastewater. Chem. Eng. J. 2020;383:123174-1–8. https://doi.org/10.1016/j.cej.2019.123174.
Roy S, Hegde M, Madras G. Catalysis for NOx abatement. Appl Energy. 2009;86:2283–97. https://doi.org/10.1016/j.apenergy.2009.03.022.
Lopes D, Zotin F, Palacio L. Copper-nickel catalysts from hydrotalcite precursors: The performance in NO reduction by CO. Appl Catal B Environ. 2018;237:327–38. https://doi.org/10.1016/j.apcatb.2018.06.007.
Okamoto Y, Gotoh H, Aritani H, Tanaka T, Yoshida S. Zirconia-supported copper catalysts for NO-CO reactions - surface copper species on zirconia. J Chem Soc Faraday Trans. 1997;93:3879–85. https://doi.org/10.1039/a704625a.
Pan KL, Young CW, Pan GT, Chang MB. Catalytic reduction of NO by CO with Cu-based and Mn-based catalysts. Catal Today. 2020;348:15–25. https://doi.org/10.1016/j.cattod.2019.08.038.
Qin Y, Huang L, Zheng J, Ren Q. Low-temperature selective catalytic reduction of NO with CO over A-Cu-BTC and AOx/CuOy/C catalyst. INOCHE. 2016;72:78–82. https://doi.org/10.1016/j.inoche.2016.08.018.
Qin Y, Huang L, Zhang D, Sun L. Mixed-node A-Cu-BTC and porous carbon based oxides derived from A-Cu-BTC as low temperature NO-CO catalyst. Inorg Chem Commun. 2016;66:64–8. https://doi.org/10.1016/j.inoche.2016.02.005.
Anbia M, Sheykhi S. Synthesis of nanoporous copper terephthalate [MIL-53(Cu)] as a novel methane-storage adsorbent. J Nat Gas Chem. 2012;21:680–4. https://doi.org/10.1016/S1003-9953(11)60419-2.
Franck M, Serre C, Férey G. Synthesis, structure determination and properties of MIL-53as and MIL-53ht: the first CrIII hybrid inorganic–organic microporous solids: CrIII(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H}. R Soc Chem. 2002;601:51–6. https://doi.org/10.1039/B201381A.
Dapaah M, Niu Q, Yu YY, You T, Liu B, Cheng L, Efficient persistent organic pollutant removal in water using MIL-metal-organic framework driven Fenton-like reactions: A critical review. Chem. Eng. J. 2021;431:134182-1–21. https://doi.org/10.1016/j.cej.2021.134182.
Bellat JP, Bezverkhyy I, Weber G, Royer S, Averlant R, Giraudon JM, Lamonier JF. Capture of formaldehyde by adsorption on nanoporous materials. J Hazard Mater. 2015;300:711–7. https://doi.org/10.1016/j.jhazmat.2015.07.078.
Damas GB, Costa LT, Ahuja R, Araujo CM. Understanding carbon dioxide capture on metal-organic frameworks from first-principles theory: The case of MIL-53(X), with X = Fe3+, Al3+, and Cu2+. J Chem Phys 2021;155:024701-1–11. https://doi.org/10.1063/5.0054874.
Ren Y, Shi M, Zhang W, Dionysiou DD, Lu J, Shan C, Zhang Y, Lv L, Pan B. Enhancing the Fenton-like catalytic activity of nFe2O3 by MIL-53(Cu) support: A mechanistic investigation. Environ Sci Technol. 2020;54:5258–67. https://doi.org/10.1021/acs.est.0c00203.
Kassem AA, Abdelhamid HN, Fouad DM, Ibrahim SA. Metal-organic frameworks (MOFs) and MOFs-derived CuO@C for hydrogen generation from sodium borohydride. Int J Hydrogen Energy. 2019;44:31230–8. https://doi.org/10.1016/j.ijhydene.2019.10.047.
Seyed Mousavi SAH, Sadrameli SM, Saeedi Dehaghani AH. Energy recovery from high density polyethylene plastic via pyrolysis with upgrading of the product by a novel nano MIL-53 (Cu) derived@Y zeolite catalyst using response surface methodology. Fuel Process. Technol. 2022;231:107257-1–19. https://doi.org/10.1016/j.fuproc.2022.107257.
Teixeira C, Montani S, Palacio L, Zotin F. The effect of preparation methods on the thermal and chemical reducibility of Cu in Cu-Al oxides. Dalt Trans. 2018;47:10989–1001. https://doi.org/10.1039/c8dt01150h.
Roisnel T, Rodríquez-Carvajal J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater Sci Forum. 2001;378–381:118–23. https://doi.org/10.4028/www.scientific.net/msf.378-381.118.
Abdelouhab S, François M, Elkaim E, Rabu P. Ab initio crystal structure of copper(II) hydroxy-terephthalate by synchrotron powder diffraction and magnetic properties. Solid State Sci. 2005;7:227–32. https://doi.org/10.1016/j.solidstatesciences.2004.10.020.
Kraus W, Nolze G. POWDER CELL—a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J Appl Cryst. 1996;29:301–3. https://doi.org/10.1107/S0021889895014920.
Kuśtrowski P, Chmielarz L, Bozek E, Sawalha M, Roessner F. Acidity and basicity of hydrotalcite derived mixed Mg-Al oxides studied by test reaction of MBOH conversion and temperature programmed desorption of NH3 and CO2. Mater Res Bull. 2004;39:263–81. https://doi.org/10.1016/j.materresbull.2003.09.032.
Nyquist RA, Kagel RO. Infrared Spectra of Inorganic Compounds. New York: Academic press; 1971.
Yu J, Wang J. Thermal decomposition behavior of terephthalate in inert gas. Chin Pet Process Pe Technol. 2017;19:1–8.
Pranger L, Goldstein A, Tannenbaum R. Competitive self-assembly of symmetrical, difunctional molecules on ambient copper surfaces. Langmuir. 2005;21:5396–404. https://doi.org/10.1021/la050508l.
Anbia M, Sheykhi S. Preparation of multi-walled carbon nanotube incorporated MIL-53-Cu composite metal-organic framework with enhanced methane sorption. J Ind Eng Chem. 2013;19:1583–6. https://doi.org/10.1016/j.jiec.2013.01.026.
Cendrowski K, Skumial P, Spera P, Mijowska E. Thermally induced formation of zinc oxide nanostructures with tailoring morphology during metal organic framework (MOF-5) carbonization process. Mater Des. 2016;110:740–8. https://doi.org/10.1016/j.matdes.2016.08.043.
Halder A, Lee S, Yang B, Pellin MJ, Vajda S, Li Z, Yang Y, Farha OK, Hupp JT. Structural reversibility of Cu doped NU-1000 MOFs under hydrogenation conditions. J. Chem. Phys. 2020;152:084703-1–7. https://doi.org/10.1063/1.5130600
Kim JY, Rodriguez JA, Hanson JC, Frenkel AI, Lee PL. Reduction of CuO and Cu2O with H2: H Embedding and Kinetic Effects in the Formation of Suboxides. J Am Chem Soc. 2003;125:10684–92. https://doi.org/10.1021/ja0301673.
Yoon JW, Seo YK, Hwang YK, Chang JS, Leclerc H, Wuttke S, Bazin P, Vimont A, Daturi M, Bloch E, Llewellyn PL, Serre C, Horcajada P, Grenèche JM, Rodrigues AE, Férey G. Controlled reducibility of a metal-organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew Chemie - Int. 2010;49:5949–52. https://doi.org/10.1002/anie.201001230.
Yang Y, Dong H, Wang Y, He C, Wang Y, Zhang X. Synthesis of octahedral like Cu-BTC derivatives derived from MOF calcined under different atmosphere for application in CO oxidation. J Solid State Chem. 2018;258:582–7. https://doi.org/10.1016/j.jssc.2017.11.033.
Scherrer P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgensrahlen. Nachr Ges Wiss Goettingen Math-Phys K. 1918;26:98–100.
Acknowledgements
This research used facilities of the Brazilian Synchrotron Light Laboratory (LNLS), part of the Brazilian Center for Research in Energy and Materials (CNPEM), a private non-profit organization under the supervision of the Brazilian Ministry for Science, Technology, and Innovations (MCTI). The XPD beamline staff is acknowledged for the assistance during the experiments (Proposal Number 20190118). We thank FAPERJ [Grant Number E-26/010.101231/2018 (ref. 210.359/2018)] for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. We thank the Laboratory of Macromolecules and Colloids Applied to the Petroleum Industry (LMCP) of IMA/UFRJ for the CHN analysis. F. M. Z. Zotin thanks CNPq for a productivity research scholarship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study, conception and design. Material preparation, data collection and analysis were performed by SSM. The first draft of the manuscript was written by LAP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Montani, S., de Lima, J.F., Zanon Zotin, F.M. et al. Thermal stability of copper-based MOF under different atmospheres. J Therm Anal Calorim 148, 119–131 (2023). https://doi.org/10.1007/s10973-022-11769-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11769-8