Abstract
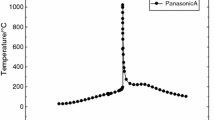
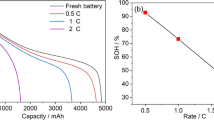
Thermal runaway phenomena of the Panasonic 21,700 LiNi0.8Co0.15Al0.05O2 lithium-ion batteries with 100, 50 and 25% capacity were studied under thermal abuses. Characteristic data of onset temperature, crucial temperature, maximum self-heat rate, maximum temperature and maximum pressure are determined and affirmed for hazard analysis. The maximum temperature could be as high as 1200 °C exceeding the auto-ignition temperature of electrolyte to ignite the flammable vapors exposed to the air. Maximum self-heat rates are determined to be as high as 64,536 °C min−1. Runaway behaviors with respect to the capacities of 50% and 25% are performed in comparison with those of 100%. Thermal runaway consequences possessed by the Panasonic 21,700 LiNi0.8Co0.15Al0.05O2 with the capacity of 25% cannot be indiscreet because its maximum temperature is approximately 600 °C with a maximum self-heat value of 10,000 °C min−1 and an 110 mmol non-condensable gases generated. Differences in runaway behaviors are compared between the 21,700 and 18,650 LiNi0.8Co0.15Al0.05O2 batteries with the same capacity of 100%.






Similar content being viewed by others
Abbreviations
- AIT:
-
Auto-ignition temperature
- ARC:
-
Accelerating rate calorimeter
- DEC:
-
Diethyl carbonate
- DMC:
-
Dimethyl carbonate
- EC:
-
Ethylene carbonate
- ESS:
-
Energy storage system
- EV:
-
Electric vehicle
- HEV:
-
Hybrid electric vehicle
- LFP:
-
Lithium iron phosphate
- LIB:
-
Lithium-ion battery
- MAWP:
-
Maximum allowable working pressure
- NCA:
-
Lithium nickel cobalt aluminum oxide
- NMC:
-
Lithium nickel manganese cobalt
- PC:
-
Propylene carbonate
- PHEV:
-
Plug hybrid electric vehicle
- PTCD:
-
Positive temperature coefficient device
- SEI:
-
Solid electrolyte interphase
- SHS:
-
Solar home systems
- SOC:
-
State of charge
- STOBA:
-
Self-terminated oligomers with hyper-branched architecture
- TMS:
-
Thermal management system
- C p :
-
Heat capacity (kJ kg−1 K−1)
- m :
-
Mass of LIB (kg)
- m ej :
-
Mass of the ejecta of LIB due to rupture of battery can under thermal runaway (kg)
- m rxn :
-
Mass of reactive components in an LIB (kg)
- n gas :
-
Quantity of non-condensable gases (mol)
- n vapor :
-
Quantity of vapor (mol)
- n tot :
-
Quantity of non-condensable gases and vapor (mol)
- P f :
-
Final pressure in autoclave after the thermal runaway of LIB (bar)
- P max :
-
Maximum pressure in autoclave under the thermal runaway of LIB (bar)
- Q sei :
-
Heat generation rate from SEI decomposition (Wm−3)
- Q ne :
-
Heat generation rate from reaction of lithiated anode material with electrolyte (Wm−3)
- Q pe :
-
Heat generation rate from reaction of delithiated cathode material with electrolyte (Wm−3)
- Q ele :
-
Heat generation rate from decomposition of electrolyte (Wm−3)
- T amb :
-
Ambient temperature (°C or K)
- T BD :
-
The temperature of separator breakdown (°C or K)
- T cr :
-
Crucial temperature of LIB under thermal runaway (°C or K)
- T max :
-
Maximum temperature of LIB under thermal runaway (°C or K)
- T m r :
-
Temperature with maximum self-heat rate of LIB under thermal runaway (°C or K)
- T onset :
-
Exothermic onset temperature of LIB under thermal runaway (°C or K)
- T PE :
-
Melting point of PE (°C or K)
- T PP :
-
Melting point of PP (°C or K)
- T sei :
-
Temperature of SEI decomposition (°C or K)
- T sm :
-
The temperature ranges for the action and operation of safety measures to mitigate the thermal runaway of LIBs (°C or K)
- V :
-
Void volume of the reaction vessel (m3 or mL)
- dT/dt :
-
Self-heat rate (°C min−1)
- (dT/dt)max :
-
Maximum self-heat rate (°C min−1)
- Δn :
-
Quantity of non-condensable gases ( mol)
- ΔT ad :
-
Adiabatic temperature rise of LIB under thermal runaway
- δ :
-
Conversion of reactant
- Cr:
-
Crucial
- max:
-
Maximum
References
The Royal Swedish Society of Science, Scientific background on the Nobel Prize in Chemistry 2019. https://www.nobelprize.org/prizes/chemistry/2019/goodenough/facts/ ; https://www.nobelprize.org/prizes/chemistry/2019/yoshino/facts/; https://www.nobelprize.org/prizes/chemistry/2019/whittingham/facts/
Yoshino A. The lithium-ion battery: two breakthroughs in development and two reasons for the Nobel Prize. Bull Chem Soc Jpn. 2022;95:195–7. https://doi.org/10.1246/bcsj.20210338.
Whittingham MS. Special editorial perspective: beyond li-ion battery chemistry. Chem Rev. 2020;120:6328–30. https://doi.org/10.1021/acs.chemrev.0c00438.
Zubi G, Dufo-López R, Carvalho M, Pasaoglu G. The lithium-ion battery: state of the art and future perspectives. Renew Sust Energ Rev. 2018;89:292–308. https://doi.org/10.1016/j.rser.2018.03.002.
Whittingham MS. Lithium batteries: 50 years of advances to address the next 20 years of climate issues. Nano Lett. 2020;20(12):8435–7. https://doi.org/10.1021/acs.nanolett.0c04347.
Goodenough JB. Evolution of strategies for modern rechargeable batteries. Acc Chem Res. 2013;46(5):1053–61. https://doi.org/10.1021/ar2002705.
Duh YS, Lin KH, Kao CS. Experimental investigation and visualization on thermal runaway of hard prismatic lithium-ion batteries used in smart phones. J Therm Anal Calorim. 2018;132:1677–92. https://doi.org/10.1007/s10973-018-7077-2.
Sun P, Bisschop R, Niu H, Huang X. A review of battery fires in electric vehicles. Fire Technol. 2020. https://doi.org/10.1007/s10694-019-00944-3.
Feng X, Ouyang M, Liu X, Lu L, Xia Y, He X. Thermal runaway mechanism of lithium ion battery for electric vehicles: a review. Energy Storage Mater. 2018;10:246–67. https://doi.org/10.1016/j.ensm.2017.05.013.
Ren D, Feng X, Liu L, Hsu H, Lu L, Wang L, He X, Ouyang M. Investigating the relationship between internal short circuit and the thermal runaway of lithium-ion batteries under thermal abuse conditions. Energy Stor Mater. 2021;34:563–73. https://doi.org/10.1016/j.ensm.2020.10.020.
Feng X, Zheng S, Ren D, He X, Wang L, Cui H, Liu X, Jin C, Zhang F, Xu C, Hsu H, Gao S, Chen T, Li Y, Wang T, Wang H, Li M, Ouyang M. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl Energy. 2019;246:53–64. https://doi.org/10.1016/j.apenergy.2019.04.009.
Manthiram A. A reflection on lithium-ion battery cathode chemistry. Nat Commun. 2020;11:1550–8. https://doi.org/10.1038/s41467-020-15355-0.
Huang P, Yao C, Mao B, Wang Q, Sun J, Bai Z. The critical characteristics and transition process of lithium-ion battery runaway. Energy. 2020;213: 119082. https://doi.org/10.1016/j.energy.2020.119082.
Chen H, Buston JEH, Gill J, Howard D, William RCE, Rao Vendra CM, Shelke A, Wen JX. An experimental study on thermal runaway characteristics of lithium-ion batteries with high specific energy and prediction of heat release rate. J Power Sources. 2020;472: 228585. https://doi.org/10.1016/j.jpowsour.2020.228585.
Mao N, Zhang T, Wang Z, Cai Q. A systematic investigation of internal physical and chemical changes of lithium-ion batteries during overcharge. J Power Sources. 2022;518: 230767. https://doi.org/10.1016/j.jpowsour.2021.230767.
Dubaniewicz TH, Barone TL, Brown CB, Thomas RA. Comparison of thermal runaway pressures within sealed enclosures for nickel manganese cobalt and iron phosphate cathode lithium-ion cells. J Loss Prevent Proc. 2022;76: 104739. https://doi.org/10.1016/j.jlp.2022.104739.
Xu B, Lee J, Kown D, Kong L, Pecht M. Mitigation strategies for Li-ion battery thermal runaway: a review. Renew Sust Energ Rev. 2021;150: 111437. https://doi.org/10.1016/j.rser.2021.111437.
Jin C, Sun Y, Wang H, Lai X, Wang S, Chen S, Rui X, Zheng Y, Feng X, Wang H, Ouyang M. Model and experiments to investigate thermal runaway characterization of lithium-ion batteries induced by external heating method. J Power Sources. 2021;504: 230065. https://doi.org/10.1016/j.jpowsour.2021.230065.
Shen H, Zhang Y, Wu Y. A comparative study on air transport safety of lithium-ion batteries with different SOCs. Appl Therm Eng. 2020;179: 115679. https://doi.org/10.1016/j.applthermaleng.2020.115679.
Lamb J, Orendorff CJ, Steele LA, Spangler S. Failure propagation in multi-cell lithium ion batteries. J Power Sources. 2015;283:517–23. https://doi.org/10.1016/j.jpowsour.2014.10.081.
Yuan C, Wang Q, Wang Y, Zhao Y. Inhibition effect of different interstitial materials on thermal runaway propagation in the cylindrical lithium-ion battery module. Appl Therm Eng. 2019;153:39–50. https://doi.org/10.1016/j.applthermaleng.2019.02.127.
Tang W, Tam WC, Yuan L, Dubaniewicz T, Thomas R, Soles J. Estimation of the critical external heat leading to the failure of lithium-ion batteries. Appl Therm Eng. 2020;179: 115665. https://doi.org/10.1016/j.applthermaleng.2020.115665.
Li Q, Yang C, Santhanagopalan S, Smith K, Lamb J, Steele LA, Torres-Castro L. Numerical investigation of thermal runaway mitigation through a passive thermal management system. J Power Sources. 2019;429:80–8. https://doi.org/10.1016/j.jpowsour.2019.04.091.
Said AO, Lee C, Stoliarov SI. Experimental investigation of cascading failure in 18650 lithium ion cell arrays: Impact of cathode chemistry. J Power Sources. 2020;446: 227347. https://doi.org/10.1016/j.jpowsour.2019.227347.
Zhang H, Li C, Zhang R, Lin Y, Fang H. Thermal analysis of a 6s4p Lithium-ion battery pack cooled by cold plates based on a multi-domain modeling framework. Appl Therm Eng. 2020;173: 115216. https://doi.org/10.1016/j.applthermaleng.2020.115216.
Duh YS, Tsai MT, Kao CS. Characterization on the thermal runaway of commercial 18650 lithium-ion batteries used in electric vehicle. J Therm Anal Calorim. 2017;127:983–93. https://doi.org/10.1007/s10973-016-5767-1.
Golubkov AW, Scheikl S, Planteu P, Voitic G, Wiltsche H, Stangl C, Fauler G, Thaler A, Hacker V. Thermal runaway of commercial 18650 li-ion batteries with LFP and NCA cathodes-impact of charge and overcharge. RSC Adv. 2015;5:57171–86. https://doi.org/10.1039/C5RA05897J.
Lammer M, Königseder A, Gluschitz P, Hacker V. Influence of aging on the heat and gas emissions from commercial lithium ion cells in case of thermal failure. J Electrochem Sci Eng. 2018;8(1):101–10. https://doi.org/10.5599/jese.476.
Perea A, Paolella A, Dubé J, Champagne D, Mauger A, Zaghib K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J Power Sources. 2018;399:392–7. https://doi.org/10.1016/j.jpowsour.2018.07.112.
Lammer M, Königseder A, Hacker V. Holistic methodology for characterisation of the thermally induced failure of commercially available 18650 lithium ion cells. RSC Adv. 2017;7:24425–9. https://doi.org/10.1039/C7RA02635H.
Kvasha A, Gutiérrez C, Osa U, Meatza I, Blazquez JA, Macicior H, Urdampilleta I. A comparative study of thermal runaway of commercial lithium ion cells. Energy. 2018;159(159):547–57. https://doi.org/10.1016/j.energy.2018.06.173.
Xu C, Feng X, Huang W, Duan Y, Chen T, Gao S, Lu L, Jiang F, Ouyang M. Internal temperature detection of thermal runaway in lithium-ion cells tested by extended-volume accelerating rate calorimetry. J Energy Storage. 2020;31: 101670. https://doi.org/10.1016/j.est.2020.101670.
Roth EP. Abuse response of 18650 Li-ion cells with different cathodes using EC:EMC/LiPF6 and EC:PC:DMC/LiPF6 electrolytes. ECS Trans. 2008;11(19):19–41. https://doi.org/10.1149/1.2897969.
Acknowledgements
The authors wish to thank the National Science Council, R.O.C., for financial support of this study under contract No. NSC 101–2221-E-239–017-MY3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duh, YS., Lin, YC., Ho, TC. et al. Experimental study on the runaway behaviors of Panasonic 21,700 LiNi0.8Co0.15Al0.05O2 battery used in electric vehicle under thermal failure. J Therm Anal Calorim 147, 12005–12018 (2022). https://doi.org/10.1007/s10973-022-11394-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11394-5