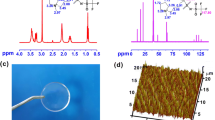
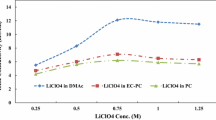
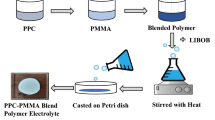
Abstract
Polymethylmethacrylate (PMMA) is a common polymer electrolyte matrix material whose pyrolysis characteristics are vital factors affecting the development of PMMA-based polymer electrolytes. In this study, four polymer electrolytes based on PMMA were prepared. Electrochemical workstation was used to verify the electrochemical performance of PMMA-based polymer electrolytes Fourier-transform infrared spectroscopy was adopted to assess the changes of pure polymer and polymer electrolyte groups, and the synchronous thermal analyzer was used to analyze the pyrolysis characteristics of PMMA-based polymer electrolytes. The apparent activation energy of PMMA-based polymer electrolyte was calculated by Kissinger method and FWO method, and the integral method Coats–Redfern was used to determine the pyrolysis mechanism function of the main pyrolysis stage. The results revealed that PMMA-based polymer electrolytes had two or three pyrolysis stages at high temperature. The PMMA composite gel polymer electrolyte had the highest thermal stability, whereas the gel polymer electrolyte had the lowest apparent activation energy. The apparent activation energy was 235.9 and 127.8 kJ mol−1 for PMMA composite electrolyte and PMMA gel polymer electrolyte, respectively. The main pyrolysis mechanism of PMMA-based polymer electrolytes was third-order reaction and three-dimensional diffusion reaction.










Similar content being viewed by others
Abbreviations
- \(A\) :
-
Pre-exponential factor (s−1)
- \(\alpha\) :
-
Degree of conversion (%)
- \(\beta\) :
-
Heat rates (°C min−1)
- \(E_{{\text{a}}}\) :
-
Apparent activation energy (kJ mol−1)
- \(f(\alpha )\) :
-
Differential form of mechanism function
- \(G(\alpha )\) :
-
Integral form of mechanism function
- \(R\) :
-
Universal gas constant (8.314 J mol−1 K−1)
- \(r\) :
-
Fitted correlation coefficient
- \(T_{0}\) :
-
Initial exothermic temperature (℃)
- \(T_{{\text{p}}}\) :
-
Maximum self-heating rate temperature (℃)
References
Wu HL, Chong YH, Ong HC, Shu CM. Thermal stability of modified lithium-ion battery electrolyte by flame retardant, tris (2,2,2-trifluoroethyl) phosphite. J Therm Anal Calorim. 2021;147:4245–52.
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci. 2011;4:3243–62.
Kawamura T, Kimura A, Egashira M, Okada S, Yamaki J. Thermal stability of alkyl carbonate mixed-solvent electrolytes for lithium-ion cells. J Power Sources. 2002;104:260–4.
Bhute MV, Kondawar SB. Electrospun poly(vinylidene fluoride)/cellulose acetate/AgTiO2 nanofibers polymer electrolyte membrane for lithium-ion battery. Solid State Ion. 2019;333:38–44.
Chung YH, Jhang WC, Chen WC, Wang YW, Shu CM. Thermal hazard assessment for three C rates for a Li-polymer battery by using vent sizing package 2. J Therm Anal Calorim. 2017;127:809–17.
Lin Y, Li J, Lai Y, Liu J. A wider temperature range polymer electrolyte for all-solid-state lithium-ion batteries. RSC Adv. 2013;3:10722–30.
Wang SH, Yan HX, Yan MX, Zhang QL. Preparation and properties of gel polymer electrolyte based on P (MMA-MAh). J Funct Mater. 2008;05:775–8.
Liu X, Zhan Y, Zhao C, Su Y, Luo Y. A novel polymer electrolyte matrix incorporating ionic liquid into waterborne polyurethane for lithium-ion battery. Polymers. 2020;12:1513–24.
Sari A, Alkan C, Kolemen U, Uzun O. Eudragit S(methyl methacrylate methacrylic acid copolymer)/fatty acid blends as form-stable phase change material for latent heat thermal energy storage. J Appl Polym Sci. 2010;101:1402–6.
Xiao Y, Li DJ, Lü HF, Shu CM. Effects of 1-butyl-3-methylimidazolium tetrafluoroborate and the oxygen concentration on the spontaneous combustion of coal. J Therm Anal Calorim. 2019;138:3445–54.
Ataollahi N, Cappelletto E, Vezzù K, Noto VD, Cavinato G, Callone E, Dirè S, Scardi P, Maggio RD. Properties of anion exchange membrane based on polyamine: effect of functionalized silica particles prepared by sol–gel method. Solid State Ion. 2018;322:85–92.
Singhal A, Dubey KA, Bhardwaj YK, Jain D, Choudhury S, Tyagi AK. UV-shielding transparent PMMA/In2O3 nanocomposite films based on In2O3 nanoparticles. Rsc Adv. 2013;3:20913–21.
Xiao Y, Guo T, Shu CM, Li DJ, Chen LG. Effects of oxygen concentrations on the coal oxidation characteristics and functional groups. J Therm Anal Calorim. 2020;142:899–912.
Zhao J, Wang T, Deng J, Shu CM, Zeng Q, Guo T, Zhang YX. Microcharacteristic analysis of CH4 emissions under different conditions during coal spontaneous combustion with high-temperature oxidation and in situ FTIR. Energy. 2020;209:118494.
Zhao J, Deng J, Chen L, Wang T, Song J, Zhang Y, Shu CM, Zeng Q. Correlation analysis of the functional groups and exothermic characteristics of bituminous coal molecules during high-temperature oxidation. Energy. 2019;181:136–47.
Liu DF, Nie J, Guan WC, Duan HQ, Zhuo LM. Characterizations of a branched ester-type lithium imide in poly(ethylene oxide)-based polymer electrolytes. Solid State Ion. 2004;167:131–6.
Khatmullina KG, Baimuratova GR, Lesnichaya VA, Shuvalova NI, Yarmolenke OV. Mechanical and electrochemical properties of new nanocomposite polymer electrolytes based on poly(vinylidene fluoride-co-hexafluoropropylene) and SiO2 addition. Polym Sci. 2018;60:222–8.
Wang W, Su W, Jiao Z, Wang Q. Thermal hazard analysis of inorganic peroxide initiators with varying water concentrations. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-10090-6.
Bai ZJ, Wang CP, Deng J, Kang FR, Shu CM. Effects of ionic liquids on the chemical structure and exothermic properties of lignite. J Mol Liq. 2020;309:113019.
Li DJ, Xiao Y, Lü HF, Xu F, Liu KH, Shu CM. Effects of 1-butyl-3-methylimidazolium tetrafluoroborate on the exothermic and heat transfer characteristics of coal during low-temperature oxidation. Fuel. 2020;273:117589.
Xu Y, Sun X, Shen R, Wang Z, Wang QS. Thermal behavior and smoke characteristics of glass/epoxy laminate and its foam core sandwich composite. J Therm Anal Calorim. 2020;141:1173–82.
Meng W, Lu Y, Carreto-Vazquez VH, Wang Q, Mannan MS. Effects of ferric oxide on decompositions of methyl ethyl ketone peroxide. J Loss Prev Proc Ind. 2012;25:202–8.
Zhang Z, Xu G, Wang Q, Cui Z, Wang L. Pyrolysis characteristics, kinetics, and evolved gas determination of chrome-tanned sludge by thermogravimetry-Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry. Waste Manag. 2019;93:130–7.
Li B, Liu G, Bi MS, Li ZB, Han B, Shu CM. Self-ignition risk classification for coal dust layers of three coal types on a hot surface. Energy. 2020;216:119197.
Naqvi SR, Uemura Y, Osman N, Yusup S. Kinetic study of the catalytic pyrolysis of paddy husk by use of thermogravimetric data and the Coats-Redfern model. Res Chem Intermed. 2015;41:9743–55.
Deng J, Zhao JY, Xiao Y, Zhang YN, Huang AC, Shu CM. Thermal analysis of the pyrolysis and oxidation behaviour of 1/3 coking coal. J Therm Anal Calorim. 2017;129:1779–86.
Wang WH, Huang Y, Hu SY, Su W, Pan Y, Shu CM. Thermal hazards analysis for benzoyl peroxide in the presence of hexanoic acid. Process Saf Environ Prot. 2022;157:208–17.
Wu H, Zheng J, Zou C, Gao Q, Wu L, Zhang C, Wen D, Wang W, Wang Y, Liu HC. Numerical analysis of kinetics of char combustion process and selection of mechanism functions. J Therm Anal Calorim. 2021. https://doi.org/10.1007/s10973-021-10749-8.
Acknowledgements
This research project was sponsored by the National Key Research and Development Program of China (No. 2018YFC0809500), National Natural Science Foundation of China (No. 51974165), and Chongqing Postgraduate Research and Innovation Project (CYS19354).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, WH., Su, W., Hu, SY. et al. Pyrolysis characteristics and kinetics of polymethylmethacrylate-based polymer electrolytes for lithium-ion battery. J Therm Anal Calorim 147, 12019–12032 (2022). https://doi.org/10.1007/s10973-022-11386-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11386-5